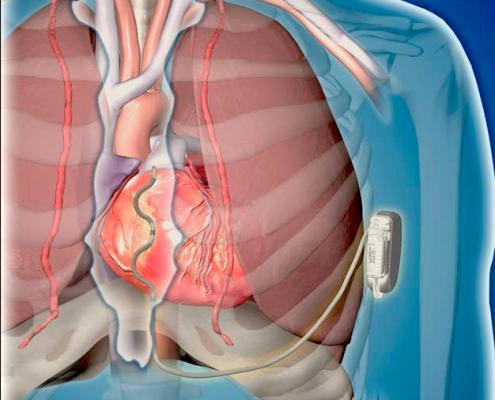
October 11, 2019 — Medtronic plc announced the start of a worldwide pivotal study evaluating its investigational Extravascular Implantable Cardioverter Defibrillator (EV ICD) system to treat dangerously fast heart rhythms. The EV ICD system is designed to deliver lifesaving defibrillation and pacing therapy via a device the same size as traditional, transvenous ICDs, but with a lead (thin wire) placed outside the heart and veins.
The first study implant procedures were performed recently by Ian Crozier, M.D., at Christchurch Hospital in Christchurch, New Zealand, and John Scherschel, M.D., at Prairie Heart Institute at HSHS St. John’s Hospital in Springfield, Ill. The principal investigator for the study is Paul Friedman, M.D., chair of the Midwest Department of Cardiovascular Medicine at Mayo Clinic, Rochester, Minn. Worldwide, the EV ICD system is investigational and not approved for sale or distribution.
“Along with the other study investigators, I am enthusiastic to evaluate the safety and efficacy of this new approach, and its potential to deliver lifesaving ICD therapy without the risks associated with leads inside the veins and heart, and without compromising on the features available in traditional ICDs,” said Bradley P. Knight, M.D., medical director of the Center for Heart Rhythm Disorders at Northwestern Medicine’s Bluhm Cardiovascular Institute at Northwestern Memorial Hospital.
The EV ICD pivotal study is a prospective, multicenter, single-arm, non-randomized, pre-market clinical study that will assess the Medtronic EV ICD system in up to 400 patients at up to 60 sites in North America, Europe, the Middle East, Asia, Australia and New Zealand.
The study’s primary effectiveness endpoint is defibrillation testing success rate at implant. The primary safety objective is freedom from major system and/or procedural complications at six months after implant. Patients will be assessed at two weeks, three months, six months and every six months thereafter.
The Medtronic EV ICD system is intended to provide the benefits of traditional, transvenous ICDs in a single system and implant procedure:
-
Lifesaving defibrillation therapy;
-
Anti-tachycardia pacing to terminate arrhythmias;
-
Post-shock pacing; and
-
Temporary, back-up, bradycardia pacing to address abnormally slow heart rates.
It is the same size (33 cc) and shape, and is expected to have similar longevity as traditional ICDs. The EV ICD device is implanted below the left armpit (in the left mid-axillary region), and the lead is placed under the sternum (breastbone). New procedure tools guide the delivery of the system.
The EV ICD pivotal study follows the successful first-in-human chronic EV ICD pilot study, which evaluated 21 patients in New Zealand and Australia and was presented as a late breaking clinical trial at Heart Rhythm 2019, as well as early research and acute feasibility studies, including the ASD (Acute Sensing and Defibrillation), SPACE2 (Substernal Pacing Acute Clinical Evaluation), and ASD2 studies.
Read the article “First-in-human Study Positive of Medtronic Extravascular ICD System”
For more information: www.medtronic.com


 January 05, 2026
January 05, 2026 









