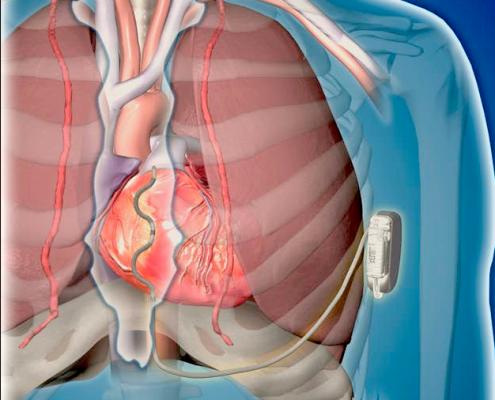
Medtronic's investigational Extravascular Implantable Cardioverter Defibrillator (EV ICD) system.
May 13, 2019 – A first-in-human pilot study of Medtronic's investigational Extravascular Implantable Cardioverter Defibrillator (EV ICD) system showed it can be implanted with no major complications, and can sense, pace and defibrillate the heart. Results from the pilot study were presented during a late-breaking session at Heart Rhythm 2019, the Heart Rhythm Society's 40th Annual Scientific Sessions.
The device uses a lead is placed outside of the heart and veins to deliver lifesaving defibrillation and anti-tachycardia pacing therapy in one system, with a device the same size as traditional, transvenous ICDs. The EV ICD system is investigational worldwide and not approved for sale anywhere.
"I am very encouraged by our pilot experience using the Medtronic EV ICD system in patients out to three months,” said Ian Crozier, M.D., Department of Cardiology, Christchurch Hospital, Christchurch, New Zealand, and principal investigator (PI) in the pilot study. “This small but important study affirms the potential of this new extravascular approach to provide pacing and lifesaving defibrillation therapy without the risks that accompany transvenous leads implanted inside the heart and veins.”
In the pilot study, 21 patients underwent the EV ICD implant procedure at four sites in New Zealand and Australia. Patients were evaluated at implant, two weeks, four to six weeks, and three months after device implant, and continue to be followed. Study investigators characterized the safety of the system and implant procedure, defibrillation effectiveness and sensing and pacing:
• At the time of implant, defibrillation testing was completed on 19 patients. The system successfully terminated induced ventricular arrhythmias in 17 patients (89.5 percent), which is consistent with prior clinical studies of existing ICDs.[1,2]
• Pacing capture was achieved in more than 95 percent of study patients.
• One patient experienced ventricular tachycardia outside the hospital setting, which was successfully detected and treated by the EV ICD system.
“The successful pilot study revealed valuable insights to inform our EV ICD clinical program, and we are eager to incorporate the learnings from this study into a pivotal trial,” said Rob Kowal, M.D., Ph.D., vice president and chief medical officer of the cardiac rhythm and heart failure division at Medtronic.
The Medtronic EV ICD system is intended to provide the benefits of traditional, transvenous ICDs including lifesaving defibrillation therapy; anti-tachycardia pacing to terminate arrhythmias; post-shock pacing to protect from sudden cardiac death; and temporary, back-up, bradycardia pacing to address abnormally slow heart rates. It is the same size (33 cc) and shape, and is expected to have similar longevity as traditional ICDs, but without any leads in the veins or heart. The EV ICD device is implanted in the left mid-axillary region below the left armpit, and the lead is placed under the sternum (breastbone). New procedure tools guide the delivery of the system.
Medtronic research teams developed the EV ICD System and have completed multiple early research and acute feasibility studies, including the ASD3 (Acute Sensing and Defibrillation), SPACE[4] (Substernal Pacing Acute Clinical Evaluation), and ASD2[5] studies. The current EV ICD pilot study continues to generate long-term data on this first-inhuman experience.
Related Content:
Medtronic Begins Pilot Study of Investigational Extravascular ICD System
Medtronic Study Confirms Feasibility of New Extravascular Approach to ICD Therapy
All the HRS 2019 late-breaking studies
References:
1. Blatt JA, Poole JE, Johnson GW, et al. No benefit from defibrillation threshold testing in the SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial). J Am Coll Cardiol. 2008 Aug 12;52(7):551-6.
2. Neuzner J, Hohnloser SH, Kutyifa V, et al. Effectiveness of single- vs. dual-coil implantable defibrillator leads: An observational analysis from the SIMPLE study. J Cardiovasc Electrophysiol. 2019 Apr 4. doi: 10.1111/jce.13943.
3. Chan JYS, Lelakowski J, Murgatroyd FD et al. Novel Extravascular Defibrillation Configuration With a Coil in the Substernal Space: The ASD Clinical Study. J Am Coll Cardiol EP 2017;3:905-10.
https://doi.org/10.1016/j.jacep.2016.12.026
4. Sholevar DP, Tung S, Kuriachan V, et al. Feasibility of extravascular pacing with a novel substernal electrode configuration: The Substernal Pacing Acute Clinical Evaluation Study. Heart Rhythm. Published online 29Nov2017. DOI:http://dx.doi.org/10.1016/j.hrthm.2017.11.030.
5. Boersma, LVA. Feasibility of Extravascular Pacing, Sensing And Defibrillation From A Novel Substernal lead: The Acute Extravascular Defibrillation, Pacing And Electrogram (ASD2) Study. Presented Heart Rhythm Society Scientific Sessions, May 11, 2018.


 January 05, 2026
January 05, 2026 









