August 9, 2018 — Medtronic plc announced the start of a pilot study of its investigational Extravascular Implantable Cardioverter Defibrillator (EV ICD) system, with the first patient implant performed at Christchurch Hospital in New Zealand. The implant represents the first intended long-term patient use of the device, which is similar in size to transvenous ICDs.
“The Medtronic EV ICD system has the potential to deliver the benefits of traditional ICDs while eliminating the risks that can occur when leads are implanted inside the veins and heart,” said Ian Crozier, M.D., Department of Cardiology, Christchurch Hospital, Christchurch, New Zealand, and principal investigator (PI) in the Medtronic Extravascular ICD Pilot Study. “We are incredibly pleased to contribute to this important research that will serve as a key step in establishing the safety and efficacy of this new approach.”
The pilot study will assess the Medtronic EV ICD system in 20 patients at four sites:
- Christchurch Hospital (Crozier);
- Austin Health in Heidelberg, Australia (PI: David O’Donnell, M.D.);
- MonashHeart in Clayton, Australia (PI: Emily Kotschet, M.D.); and
- The Prince Charles Hospital in Brisbane, Australia (PI: Haris Haqqani, M.D.).
After implantation of the system, patients and their devices will be routinely checked to assess safety and device performance.
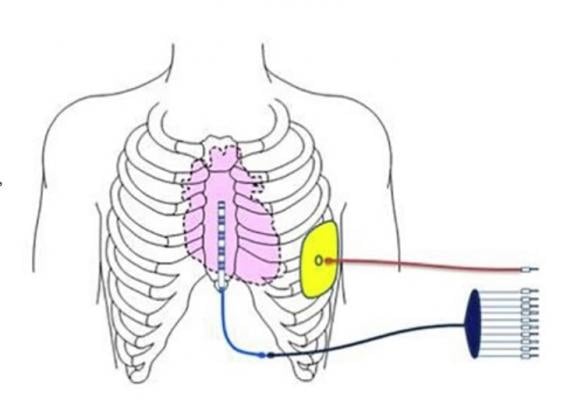
The Medtronic EV ICD system is intended to provide the benefits of traditional transvenous ICDs including lifesaving defibrillation therapy, antitachycardia pacing to painlessly terminate arrhythmias, as well as post-shock pacing to protect from sudden cardiac death; and bradycardia pacing to address abnormally slow heart rates. It also is the same size (33 cc) and shape, and is expected to have similar longevity as traditional ICDs, but without any leads (thin wires) in the veins or heart. The investigational EV ICD device is implanted in the left mid-axillary region below the left armpit, and the newly designed lead is placed under the sternum (breastbone). New procedure tools guide the delivery of the system.
Medtronic research teams developed the EV ICD System and have completed multiple early research and acute feasibility studies using the system components, including the ASD1 (Acute Sensing and Defibrillation), SPACE2 (Substernal Pacing Acute Clinical Evaluation) and ASD23 studies.
The EV ICD system is investigational and not approved for sale or distribution.
For more information: www.medtronic.com
References
3. Boersma, LVA. Feasibility of Extravascular Pacing, Sensing And Defibrillation From A Novel Substernal lead: The Acute Extravascular Defibrillation, Pacing And Electrogram (ASD2) Study. Presented Heart Rhythm Society Scientific Sessions, May 11, 2018.


 January 05, 2026
January 05, 2026 









