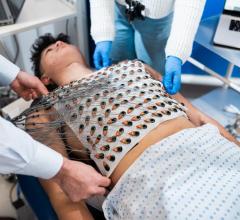
Jan. 13, 2026 — Kestra Medical Technologies, a wearable medical device and digital healthcare company, has announced a strategic collaboration with Biobeat Technologies, Ltd. to expand diagnostic ...
Sudden Cardiac Arrest
This channel includes news and new technology innovations about sudden cardiac arrest (SCA), which is also called sudden cardiac death (SCD). SCA occured when the heart suddenly ceases all electrical activity needed to keep the heart beating. Read more about the the condition from the Heart Rhythm Society. It is estimated that more than 400,000 people in the U.S. have out-of-hospital cardiac arrest each year. Cardiopulmonary resuscitation (CPR) and early defibrillation by administering an electric shock to the heart to restore its rhythm to normal are the only treatments that have been shown to improve survival after a sudden cardiac arrest (SCA). SCA is fatal in over 80 percent of cases.
Jan. 13, 2026 — Kestra Medical Technologies, a wearable medical device and digital healthcare company, has announced a ...
Medtronic has announced the commercial launch of the OmniaSecure defibrillation lead in the U.S., with first cases being ...
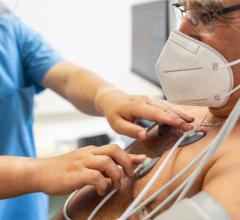
Nov. 10, 2025 — Kestra Medical Technologies, a wearable medical device and digital healthcare company, has announced ...
Pre-participation physical evaluations (PPEs) are a standard requirement for many school-aged athletes to participate in competitive sports. One purpose of the PPE is to check for any underlying cardiovascular abnormalities that could put an athlete at risk for sudden cardiac arrest (SCA), which is the number one medical cause of death in young athletes.
Sept. 9, 2025 — Neurescue ApS has received CE Mark approval for its Neurescue device, a medical device approved to treat ...
March 1, 2024 — Two new studies by Cedars-Sinai investigators support using artificial intelligence (AI) to predict ...
January 30, 2024 — Only 10% of people who experience a cardiac arrest survive.[1] In new challenge goals outlined in the ...
Sudden cardiac death (SCD) is the leading medical cause of death in young athletes and its impact is consistent worldwide. Most professional athletes in the United States are required to take part in comprehensive cardiovascular screening programs to identify often-asymptomatic congenital or inherited heart disorders, and other cardiac risk factors. There remains a debate however, whether to mandate ECGs as part of pre-participation screening programs for student athletes at the collegiate and high school levels or even at younger ages.
January 19, 2024 — Heart rhythm expert Sumeet Chugh, MD, associate director of the Smidt Heart Institute at Cedars-Sinai ...
December 19, 2023 — A vest that can map the electrical activity of the heart in fine detail could potentially be used to ...
November 17, 2023 — Sudden cardiac arrest remains a deadly and complex condition, but investigators in the Smidt Heart ...
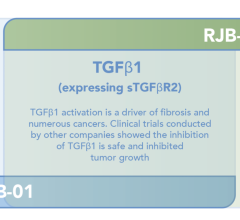
October 9, 2023 — Rejuvenate Bio announced new preclinical efficacy data for RJB-0402, a gene therapy targeting key ...
September 8, 2023 — Patients of African ancestry with dilated cardiomyopathy (DCM) are less likely to have clinically ...
August 1, 2023 —LEXEO Therapeutics, a clinical-stage gene therapy company advancing adeno-associated virus (AAV)-based ...
July 28, 2023 — GE HealthCare is recalling malfunctioning TruSignal sensors that may reduce the amount of energy sent to ...
July 12, 2023 — CPR Therapeutics Inc. (CPR-T), a Vermont-based medical device start-up company, announced today that it ...
April 6, 2023 — Atrial fibrillation (AFib) not only causes shortness of breath and palpitations but puts patients at ...





 January 14, 2026
January 14, 2026