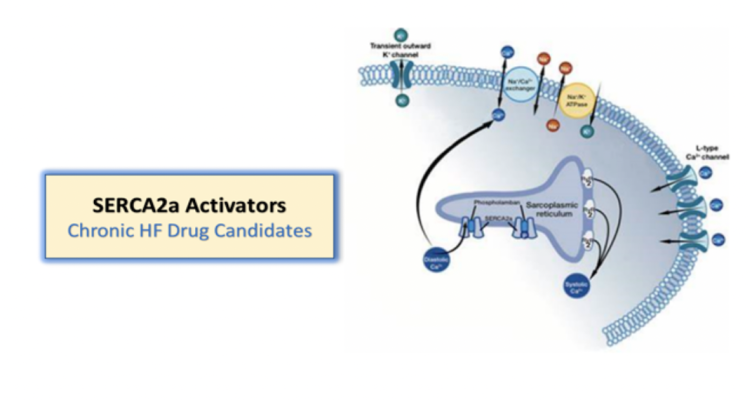
Compounds that activate SERCA2a may lead to novel therapies for heart failure
April 25, 2023 — Windtree Therapeutics, Inc., a biotechnology company focused on advancing late-stage interventions for cardiovascular disorders, announced that the European Patent Office has granted Patent No. 3599243, providing patent coverage for the dual mechanism SERCA2a Activator class of drug candidates. Windtree has preclinical drug candidates with dual mechanisms of action (inhibition of the Na+/K+ pump and activation of SERCA2a) as well as pure SERCA2a activators (devoid of action on the Na+/K+ pump). The new patent, titled: “17BETA-HETEROCYCLYL-DIGITALIS LIKE COMPOUNDS FOR THE TREATMENT OF HEART FAILURE,” provides patent protection until July 2038 for the family of compounds with a dual mechanism of action.
Like istaroxime, the intravenous late-stage development candidate for treatment of acute decompensated heart failure and cardiogenic shock, the dual mechanism compounds activate SERCA2a and inhibit the Na+/K+ pump. However, these product candidates are intended to be both oral and IV therapies and may represent a hospital inpatient therapy for acute decompensated heart failure as well as outpatient, oral therapy for hospital discharge and chronic heart failure treatment.
“SERCA2a activity is decreased in heart failure. Windtree believes therapies that activate SERCA2a represent a potential important advancement in heart failure treatment for patients,” said Craig Fraser, CEO and President of Windtree Therapeutics. “The development strategy with these dual mechanism compounds is for potential ‘fast follow-on’ drug candidates that can leverage the experience with istaroxime and provide the potential added feature of oral bioavailability for use as a treatment for chronic heart failure. Compounds that activate SERCA2a have been aggressively pursued as much sought-after targets in the treatment of heart failure, and Windtree has found a unique mechanism that activates SERCA2a.”
About Dual Mechanism SERCA2a Activators
These compounds activate SERCA2a and inhibit the Na+/K+ pump. Windtree Therapeutic’s research program is evaluating these preclinical product candidates, including oral and intravenous SERCA2a activator heart failure compounds.
About Istaroxime
Istaroxime is a first-in-class dual mechanism therapy designed to improve both systolic and diastolic cardiac function. Istaroxime is a positive inotropic agent that increases myocardial contractility through inhibition of Na+/K+- ATPase with a complimentary mechanism that facilitates myocardial relaxation through activation of the SERCA2a calcium pump on the sarcoplasmic reticulum enhancing calcium reuptake from the cytoplasm. Data from multiple Phase 2 studies in patients with acute heart failure (AHF) demonstrate that istaroxime infused intravenously significantly improves cardiac function and blood pressure without increasing heart rate or the incidence of cardiac rhythm disturbances.
For more information: https://windtreetx.com/
Related Content:
Windtree Therapeutics Announces Issuance of New Istaroxime Patent from U.S. Patent and Trademark Office
Windtree Therapeutics Announces Istaroxime Late Breaker Abstract from its Positive Phase 2 Study in Early Cardiogenic Shock (SEISMiC) to be Presented at Heart Failure Society of America
Windtree Completes Enrollment of Phase 2 Study of Istaroxime in Early Cardiogenic Shock
Windtree Finds Cardiogenic Shock Has High Cost of US Patient Care and Plans Innovation with the Unique Profile of Istaroxime


 January 28, 2026
January 28, 2026 









