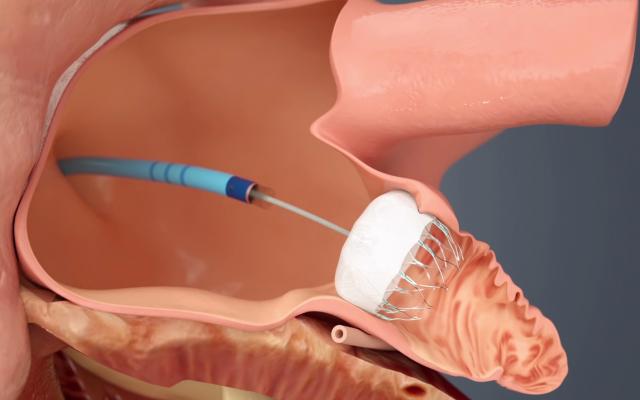
The Watchman LAA occluder being implanted in the left atrial appendage via a delivery catheter through a transseptal puncture.
The long-awaited U.S. Food and Drug Administration (FDA) approval of the first transcatheter left atrial appendage (LAA) occluder in March is seen by many cardiologists as a disruptive technology in the management of stroke risk in some atrial fibrillation (AF) patients. The FDA approval of Boston Scientific’s Watchman device offers a new stroke risk reduction option for high-risk patients with non-valvular AF who are seeking an alternative to long-term warfarin anticoagulant therapy.
“I think Watchman is a paradigm shift for how we treat high-risk patients and patients whose quality of life is impacted because they are taking anticoagulation therapy. These patients have an alternative now,” said Ramon Quesada, M.D., FACC, FACP, FSCAI, FCCP, medical director of interventional cardiology and research at Baptist Cardiac and Vascular Institute, Baptist Hospital of Miami “It will not replace warfarin or other anticoagulants, there will be patients where you don’t want to use this device,” Quesada said. “It’s not a replacement for anticoagulation therapy, it is an alternative.”
The FDA cleared the device in March for use in the United States. It had previously received CE mark in Europe in 2005.
The LAA has long been implicated as the primary source of thrombus formation in the heart, caused by AF. By sealing off the LAA, the patient can be taken off of anticoagulation therapy.
Warfarin is the most common anticoagulant prescribed to reduce stroke risk in AF patients. However, the agent is not well tolerated by all patients and has significant risk for bleeding complications. It also has a very narrow therapeutic window that can be affected by diet and other factors, requiring close monitoring with bi-weekly blood tests.
Previously, the LAA could be closed surgically, by minimally invasive surgical clipping of the appendage on the surface of the heart with AtriCure’s AtriClip device, or by using Amplatzer transcatheter septal occluders off-label. The Watchman allows LAA closure using a cath lab transcatheter procedure, which takes about 45 minutes to an hour to perform.
"The Watchman device is an important step forward in stroke management for patients with AF," said Vivek Reddy, M.D., director of the Cardiac Arrhythmia Service at the Mount Sinai Medical Center and co-principal investigator of the PROTECT AF and PREVAIL studies in a statement. "We know that up to 40 percent of patients who are eligible for oral anticoagulation do not take it for numerous reasons, highlighting the need for additional treatment options. The Watchman device is a breakthrough treatment providing those patients who are suitable for warfarin with an implant-based alternative to long-term warfarin therapy while still reducing the risk of stroke."
Solving Noncompliance and Ideal Patients
Quesada said about 60 percent of patients who should be on anticoagulants are not taking their medication. This noncompliance is usually due to bleeding complications, especially with major internal bleeds from ulcers. LAA occlusion is ideal for these types of patients.
“The elderly really benefit and do better on Watchman, because the drugs make them bleed for a lot of reasons.” Quesada said. “The higher the CHADS score, the better they do with Watchman compared to anticoagulation therapy.”
In his experience at Baptist, Quesada said patient response to the Watchman device has been mixed, with younger patients usually favoring the procedure and older patients often being more cautious, preferring to avoid any type of procedure and remain on anticoagulation therapy.
“The patients who receive the device would rather have this safety net to protect them from having a stroke, without the need to take a drug,” he said.
Younger patients often view taking a daily anticoagulant as an inconvenience and say it impacts their quality of life, since they are prevented from participating in contact sports or other activities that might results in bruising or cuts.
The FDA indicated the device to reduce the risk of thromboembolism from the LAA in patients with non-valvular atrial fibrillation who are:
1. At increased risk for stroke and systemic embolism based on CHADS2 or CHA2DS2-VASc scores;
2. Are deemed by their physicians to be suitable for warfarin; and
3. Have an appropriate rationale to seek a non-pharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device compared to warfarin.
Post Implant LAA Occlusion Protocol
At Baptist, patients remain on anticoagulation therapy for 45 days to allow endothelialization of the Watchman’s fabric covering. At 45 days, the patient undergoes a cardiac echo exam to make sure the device has not migrated or attached vegetation. If clear, the patient is taken off anticoagulants and maintained on clopidogrel and 81 mg aspirin for six months, and aspirin only thereafter.
Physician Training Needed To Avoid Complications in LAA Occlusion
One of the issues the FDA saw with the Watchman was the high rate of implant procedure-related complications, including perforations and pericardial effusions. In the initial FDA pivotal trial, PROTECT AF, the procedural failure rate was about 9 percent, with a device-related complication rate of 8.7 percent. Most of these events were at the start of the trial, but as operators became more experienced with the procedure, the rates went down by the end of the study, Quesada said. However, the FDA review panel had concerns and required a second trial, PREVAIL, to prove the procedure is teachable and the lower procedural complication rates can be maintained across various hospitals and with new operators. PREVAIL had a device-related complication rate of 4.4 percent, cutting the original trial's rate by nearly half. The implant success rate overall rose to 95 percent.
“This is not a casual procedure where you can do one of these procedures every six months and be proficient at it,” Quesada said. “You need to be all in and to do these procedures all the time. It’s not a producer for everyone. For the interventional cardiologist, it is a jump from the coronaries to structural heart procedures. If you put the time and effort in and learn the procedure and the anatomy you can do this procedure.”
He said it takes about 25 Watchman procedures to become proficient with the device implant procedure. Quesada recommends being proctored by an experienced Watchman operator because it will greatly shorten the learning curve.
He said operators need to be proficient with left side catheterizations and transseptal punctures. In addition to angiographic imaging, Watchman procedures are guided using 2-D or 3-D transesophageal echo (TEE).
Clinical Data Supporting LAA Occlusion
Reddy served as co-principal investigator for both national clinical trials. He said the results of these trials revealed that the device offers an efficient alternative to warfarin, and prevents strokes just as well. Patients with the device implanted also had a 60 percent reduction in cardiovascular mortality and 34 percent reduction in all-cause mortality.
The prospective, randomized PREVAIL trial enrolled 407 patients at 41 sites and compared the Watchman to warfarin in high-risk patients with nonvalvular AF eligible for long-term warfarin therapy. PREVAIL built on data from the PROTECT AF clinical trial, which enrolled 707 randomized patients treated with either the Watchman device or standard warfarin therapy, to evaluate the safety and effectiveness of the device. The PREVAIL trial was designed to confirm the results of the PROTECT AF trial and validate the safety of the implant procedure, including at least 25 percent of subjects treated by new operators.
Data demonstrated an increase in implant success rate overall (95 percent), and with new operators (93.2 percent), compared to PROTECT AF (90.9 percent). The overall seven-day serious procedure/device-related complication rate was 4.4 percent in PREVAIL vs. 8.7 percent in PROTECT AF, a 49 percent relative reduction.
A key result of the PREVAIL trial was that pericardial effusions requiring intervention occurred at a rate comparable to other left atrial procedures. PREVAIL reported a 1.9 percent event rate vs. 4 percent in PROTECT AF, a 52 percent relative reduction. Additionally, new operators had only one occurrence (1 percent) of pericardial effusion requiring intervention with no device embolization, peri-procedural strokes or cardiac perforation.
Operators in the Watchman trials found anatomical shaped predicted outcomes, with different shaped LAAs more prone to emboli formation. Quesada said cauliflower-shaped LAAs were found to be the most prone to clot formation in AF patients.
Related LAA Occlusion Content:
Occluding the Left Atrial Appendage (LAA)
VIDEO: New Data on LAA Occlusion From the PREVAIL and PROTECT Trials — Interview with Vivek Reddy, M.D.
VIDEO: Comparison Between Watchman vs. Amulet LAA Occluders — Interview with Ashish Pershad, M.D.
Mixed Results in PREVAIL Trial Comparing Five-Year Data for LAA Closure Compared to Warfarin


 December 19, 2025
December 19, 2025 









