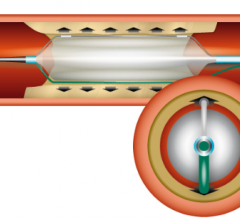
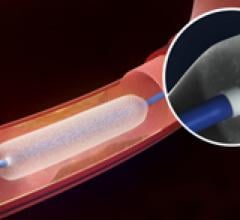
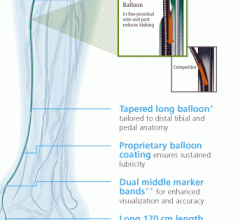
Balloon Catheter
This channel includes news and new technology innovations for angioplasty balloon catheters (PTA). These are used in arteries with atherosclerotic lesions to compress the plaque expand the artery lumen to reopen occluded or heavily stenosed atherosclerotic lesions. Balloons are often used in combination with a stent to prop the treated vessel segment open. In addition to plain old balloon angioplasty (POBA), this section includes news about drug-coated balloon (DCB), valvuloplasty balloons and specialty cutting balloon.

October 25, 2013 – The global sales of drug-eluting balloons (DEBs) across the 10 major markets (10MM) are expected to ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...

August 19, 2013 — Medtronic Inc. announced the submission of its first pre-market approval (PMA) module to the U.S. Food ...
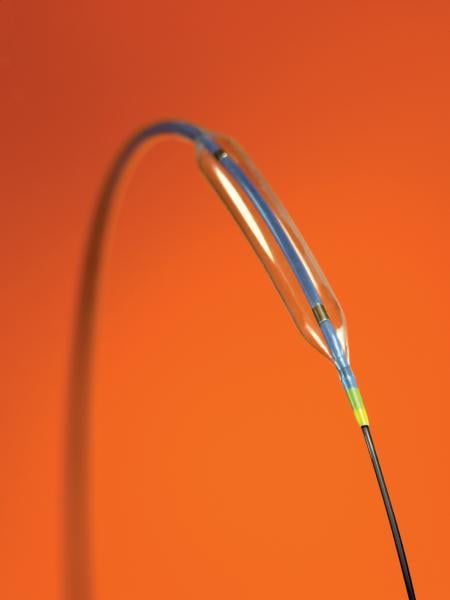
Percutaneous transluminal coronary angioplasty (PTCA) balloon catheters were the first devices used in the field of ...
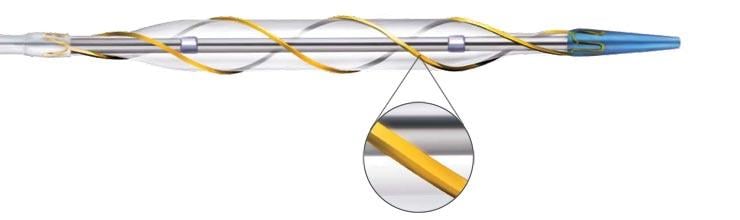
July 23, 2013 — AngioScore Inc. announced the completion of enrollment in the Drug-Coated AngioSculpt Scoring Balloon ...
July 1, 2013 — One-year data from an Italian multicenter randomized controlled trial of the In.Pact Falcon drug-eluting ...
June 25, 2013 — Cordis Corp. announced the European CE marking and U.S. Food and Drug Administration (FDA) approval of ...
June 20, 2013 — Biosensors International Group Ltd. has entered into a licensing agreement with Eurocor GmbH, a group ...
June 10, 2013 — C. R. Bard Inc. announced the enrollment of the first patient into the Lutonix Below the Knee (BTK) Clin ...
June 4, 2013 — Covidien recently introduced its RapidCross 0.014-inch Rapid Exchange Percutaneous Transluminal ...

Interventional cardiology treats cardiovascular disease, an illness currently afflicting one in every three Americans ...
May 1, 2013 — InterValve Inc. announced that it has received 510(k) clearance to market the new V8 Aortic Valvuloplasty ...
February 22, 2013 — Physicians have new tools for treating coronary chronic total occlusions (CTOs) after Abbott ...

 November 01, 2013
November 01, 2013