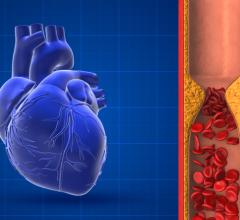
June 13, 2024 — The U.S. Food and Drug Administration (FDA) announced that Teleflex, and their subsidiary Arrow International, are recalling the Arrow FiberOptix Intra-Aortic Balloon Catheter Kit and ...
Balloon Catheter
This channel includes news and new technology innovations for angioplasty balloon catheters (PTA). These are used in arteries with atherosclerotic lesions to compress the plaque expand the artery lumen to reopen occluded or heavily stenosed atherosclerotic lesions. Balloons are often used in combination with a stent to prop the treated vessel segment open. In addition to plain old balloon angioplasty (POBA), this section includes news about drug-coated balloon (DCB), valvuloplasty balloons and specialty cutting balloon.
Aug. 21, 2025 — Boston Scientific has initiated the AGENT DCB STANCE trial to assess the safety and effectiveness of the ...
July 31, 2024 — BioCardia, Inc., a developer of cellular and cell-derived therapeutics for the treatment of ...
June 18, 2024 — Elixir Medical has announced the company’s novel bioadaptive implant, DynamX Sirolimus-Eluting Coronary ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
June 13, 2024 — The U.S. Food and Drug Administration (FDA) announced that Teleflex, and their subsidiary Arrow ...
June 4, 2024 — A patient at HonorHealth Research Institute is one of the nation’s first — and the first in Arizona and ...
April 4, 2024 — The U.S. Food and Drug Administration (FDA) announced that Medos International Sàrl is recalling Cerenov ...
April 2, 2024 — Medical device technology developer Concept Medical has announced it has been granted Investigational ...
March 20, 2024 — PECA Labs, a medical device company reimagining the field of vascular grafts and valves with durable ...
March 20, 2024 — Biotronik has been granted Breakthrough Device Designation (BDD) from the US Food and Drug ...
March 13, 2024 — In the largest randomized clinical trial and first of its kind to date in the United States, a team led ...
March 1, 2024 — Boston Scientific Corporation today announced it has received U.S. Food and Drug Administration (FDA) ...
February 9, 2024 — BIOTRONIK introduced the Micro Rx catheter, a novel rapid exchange microcatheter developed to easily ...
January 18, 2024 — Summa Therapeutics, LLC announced that the first-in-man injectable angioplasty procedures for ...
January 4, 2024 — Laguna Tech USA, a privately-held medical technology company dedicated to innovations in structural ...
November 1, 2023 — The VIVA Foundation has announced the results for the first Late-Breaking Clinical Trial sessions at ...




 August 22, 2025
August 22, 2025