September 20, 2018 — Cardiac Dimensions announced the company has randomized its first patient in the CARILLON Pivotal Trial.
The CARILLON Trial is evaluating the Carillon Mitral Contour System for the treatment of functional mitral regurgitation (FMR) associated with heart failure (HF) as compared to a randomized control group treated with optimal medical management according to established heart failure guidelines. The multi-center, double-blinded, randomized controlled trial is expected to randomize 450 patients at up to 75 centers in North America and Europe. The trial has primary safety and efficacy endpoints at 12 months and will follow the randomized patients out to five years to document long-term safety and clinical status. The CARILLON Trial also includes a cross-over feature that allows patients originally randomized to the control group to receive the Carillon System after their one-year follow-up visit.
“Current treatments to improve the quality of life for patients with FMR fall short and many heart failure patients are too frail for open-heart surgery,” said Samir Kapadia, M.D., interventional cardiologist and cath lab director at the Heart & Vascular Institute, Cleveland Clinic, and one of the principal investigators of the CARILLON Trial. “Transcatheter treatments have been shown to be safe and effective in the treatment of valve diseases, but there is no minimally invasive intervention yet approved in the U.S. for these patients. Today, most FMR patients are treated with medical therapy only for decreasing symptoms but this does not address the underlying anatomical problem leading to mitral regurgitation. The CARILLON Trial should provide us a better understanding of the benefits of the Carillon System as an option for heart failure patients with FMR.”
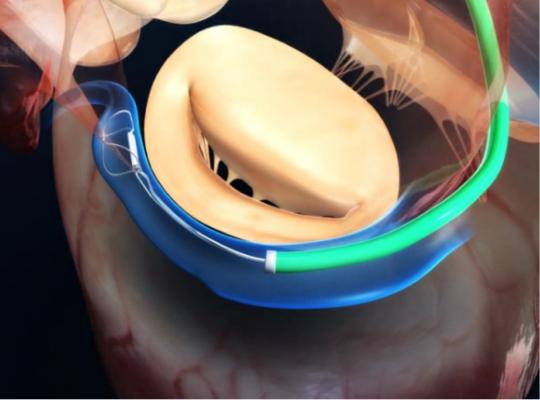
Functional mitral regurgitation occurs when the left ventricle of the heart is enlarged, dilating (stretching) the valve opening (annulus) and causing a backward flow of blood into the atrium. Left untreated, FMR contributes to heart failure – a chronic, progressive condition that weakens the heart and makes everyday activities difficult. The Carillon System addresses the underlying mechanical problem of FMR with a catheter-based alternative to medications and invasive surgery. Unlike other mitral regurgitation therapies, the Carillon System replicates traditional surgical standards through a minimally invasive approach that offers patients annular reduction, while keeping adjunctive therapy options open.
Commercially, the Carillon System has its CE Mark and is available in certain European markets as well as other key geographies including Turkey. Clinical data from three completed studies of the Carillon System (AMADEUS, TITAN, and TITAN II) were the basis for CE marking demonstrating safety and performance. In addition, the company will announce the results of its landmark REDUCE FMR Trial — the first randomized, blinded evaluation of a therapy for FMR — at the Transcatheter Cardiovascular Therapeutics (TCT) 2018 conference, Sept. 21-25 in San Diego.
Prof. Tomasz Siminiak, professor of cardiology at Poznan University of Medical Sciences in Poznań, Poland, randomized the first patient in the CARILLON Trial.
“We are very excited to participate in this landmark trial to study the Carillon System for patients with FMR,” said Siminiak. “These patients need access to less invasive treatment options, and to be able to contribute to advancements that have the potential to slow the progression of this chronic disease, is very important to us.”
An estimated 26 million people suffer from heart failure worldwide[1] and of those, approximately 70 percent have FMR. In the U.S., an estimated 2 million people are affected by symptomatic FMR associated with HF.[2],[3] Overall, HF is a significant clinical and economic burden with direct and indirect costs expected to grow to $70 billion by 2030.[4]
For more information: www.cardiacdimensions.com
References


 January 05, 2026
January 05, 2026 









