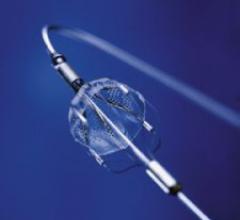
The results of Boston Scientific's pivotal SPIRIT III clinical trial reaffirmed prior safety and efficacy data ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
Boston Scientific Corp. announced two-year results from the TAXUS V In-Stent Restenosis (ISR) trial, demonstrating ...
The FDA has granted clearance to the Cordis Endovascular division of Cordis Corp. to market its PRECISE RX ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
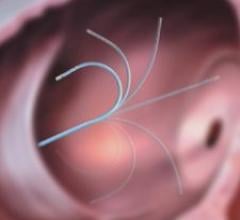
Cordis Corp. introduced the REGATTA Steerable Guidewire family of devices in the U.S. this week, which 12 ...
When used in conjunction with the ev3 embolic protection device, ev3’s PROTÉGÉ RX Carotid Stent has been FDA ...
McKesson has announced it has expanded its distribution agreement with Toshiba America Medical Systems Inc., to ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
The Vanguard Dx Angiographic Catheter with patented RadiantFlow tip technology delivers contrast in a unique way ...

The modern world of medicine has been revolutionized by the inception of minimally invasive interventional techniques ...
The TandemHeart system provides up to five liters per minute of blood flow through a cardiac catheterization procedure ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
The Vanguard Dx Angiographic Catheter with patented RadiantFlow tip technology delivers contrast in a unique way ...
A new ablation catheter designed to give physicians improved range of motion and ease of use during procedures to ...
When used in conjunction with the ev3 embolic protection device, ev3’s PROTÉGÉ RX Carotid Stent has been FDA cleared for ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
March 19, 2007 — Onset Medical Corp. has announced it has received FDA clearance to begin marketing the SoloPath ...
March 13, 2007 — Cardica Inc. announced today it has received $1.25 million in payments from Cook Medical for ...
March 9, 2007 — New studies show that about 60 percent of drug-eluting stents are used in off-label indications in the U ...

 March 27, 2007
March 27, 2007