June 13, 2007 - Cordis Endovascular announced at the 61st Annual Meeting of the Society for Vascular Surgery the ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
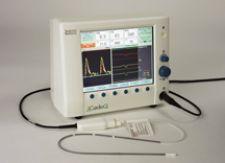
The company offers a monitor that uses a continuous wave doppler that can look right down into the aortic valve ...
June 4, 2007 — Contrary to recent studies, proper use of a drug called aprotinin to reduce bleeding during heart ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
June 4, 2007 — Biodegradable magnesium stents have been developed to treat blockages in coronary arteries. The ...
June 1, 2007 — Abbott announced it has completed its application to the U.S. Food and Drug Administration (FDA) ...
May 31, 2007 - The American Heart Association is launching a community-based program to reduce the toll of the ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
May 30, 2007 -- As concern and investigation continue concerning the link between drug-eluting stents and late-stent ...
May 29, 2007 — Stentys says the results of preclinical testing of its bifurcated stent were reported at last week's ...
May 29, 2007 — Boston Scientific Corp. has received CE Mark for its TAXUS Liberte Long paclitaxel-eluting coronary stent ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
May 29, 2007 — The FDA has cleared the Mynx VCS vascular closure system (VCD) by AccessClosure designed to seal a ...
May 29, 2007 — The FDA has cleared the CORDIS ENTERPRISE Vascular Reconstruction Device and Delivery System under ...
May 29, 2007 — Abbott announced positive results last week from ABSORB, the world’s first clinical trial evaluating the ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
May 29, 2007 — Medtronic Inc. has enrolled the first patient in the landmark PROTECT clinical study, the largest ...
May 23, 2007 — InspireMD has announced this week the enrollment of 19 patients in its First in Man study in a ...
May 23, 2007 — XTENT Inc. announced positive six-month follow-up data from the CUSTOM II clinical trial, which assessed ...

 June 12, 2007
June 12, 2007


