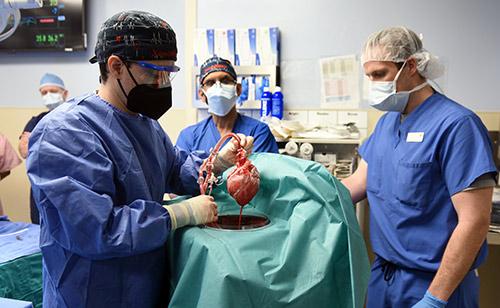
March 3, 2022 – The University of Maryland School of Medicine’s Institute of Human Virology, a Global Virus Network (GVN) Center of Excellence, physician researchers played a collaborative role in last month’s successful transplant of a genetically-modified pig heart into a patient with terminal heart disease by creating pathogen surveillance strategies and developing an infection prevention strategy for this unprecedent significant medical advancement.
“Infectious disease complications are always a concern in the field of organ transplantation, whether it is infections related to the recipient or the donor, which in this case remarkably happens to be a pig,” said Kapil Saharia, MD, MPH, Assistant Professor of Medicine in the Institute of Human Virology at the University of Maryland School of Medicine and Chief of Solid Organ Transplant Infectious Diseases Service at the University of Maryland Medical Center. “We are excited to work synergistically on this first-of-its-kind transplant through innovating the laboratory assay and protocols which allow for the surveillance of potential pig donor-derived infections.”
To decrease the risk of infection, the donor pig was raised in a disease-free, laboratory environment and screened for many known pig pathogens before being brought to the laboratory. Although all pigs are known to have the porcine endogenous retrovirus, researchers had not detected any transmission to humans or to non-human primates in earlier studies.
Procedures that transfer tissue or organs from one kind of animal to another are known as xenotransplants. The Cardiac Xenotransplantation Program at UMSOM, led by Bartley Griffith, MD, the Thomas E. and Alice Marie Hales Distinguished Professor in Transplant Surgery at UMSOM, and Muhammad M. Mohiuddin, MD, Professor of Surgery at UMSOM, tapped into the world-renowned Institute to preemptively minimize any possible risk of potential infection.
“The quality of IHV support for our experimental surgery has enabled us greatly,” said Dr. Griffith. “Our pre-op preparation and post op monitoring for pathogens has been a pathway of discovery and meaningful treatment.”
Dr. Mohuiddin said, “Although the evidence is lacking, there is a definite concern of porcine pathogens causing disease in humans. We will continue to carefully monitor the patient with the help of IHV for the zoonotic diseases.”
Robert C. Gallo, MD, The Homer & Martha Gudelsky Distinguished Professor in Medicine and Co-Founder and Director at the Institute of Human Virology at UMSOM, and GVN Co-Founder and International Scientific Director said, “Almost four years ago, the xenotransplant group came to us at the Institute of Human Virology for our expertise, particularly related to human retroviruses not unlike the one in pigs.” Dr. Gallo is world renowned for his discovery of the first human retroviruses.
Using what other researchers have published on the pig retrovirus, researchers at the Institute of Human Virology developed an in-house PCR test that will be used to screen for the virus in the organ recipient. The test will be used for surveillance of healthcare workers for exposure to this retrovirus over the coming months. The test will also be used for the research animal studies needed to advance this procedure to eventual clinical trials. These infectious disease physicians will monitor the patient for any signs of other opportunistic infection from taking immune suppressants, as well.
As a prerequisite of FDA emergency authorization, the team put together a hospital infection prevention plan for the University of Maryland Medical Center. The physicians designing the program included Dr. Saharia, Anthony Harris, MD, MPH, Professor of Epidemiology & Public Health and Division Head of Health Care Outcomes Research at the University of Maryland School of Medicine; Surbhi Leekha, MBBS, MPH, Associate Professor Epidemiology & Public Health at University of Maryland School of Medicine and Medical Director of Infection Prevention and Hospital Epidemiology at University of Maryland Medical Center; and Michelle Harris Williams, Director of Infection Prevention at University of Maryland Medical Center.
“Since this xenotransplant was performed as a compassionate lifesaving surgery, it was challenging to develop workflows to minimize risk to our healthcare providers and hospital staff, as well as other patients,” said Dr. Saharia. “We have no precedent for a xenotransplant in a clinical environment, so we worked closely with our own infection control epidemiologists to put together a plan that was safe for everyone involved.”
The infection prevention plan used disposable equipment when possible and stringent protocols for disinfection. Additionally, healthcare works are being instructed to use enhanced contact precautions when caring for the patient, which includes wearing gloves, gowns, and appropriate hand hygiene, as well as face masks and eye protection due to the ongoing COVID-19 pandemic. To further reduce risk, patient samples are hand delivered to the lab and handled in a manner similar to other highly infectious agents.
“We are pleased to be part of a team lead by Drs. Mohiuddin and Griffith for the past several years. This is certainly an important milestone in the history of organ transplantation,” said Shyam Kottilil, MBBS, PhD, Professor of Medicine, Director of the Division of Infectious Diseases in the Department of Medicine, and Director of the Division of Clinical Care and Research at the Institute of Human Virology at the University of Maryland School of Medicine and a Senior Scientific Advisor at the GVN. “We will continue to work hand-in-hand with the team to ensure safety and enhance clinical outcomes of this patient and others in the future.”
Anthony Amoroso, MD, Professor of Medicine, Associate Chief of Infectious Diseases, and Chief of Clinical Care Programs for the Institute of Human Virology at the University of Maryland School of Medicine, said, “This is very exciting that we are able to work collaboratively to support a pioneering accomplishment of Drs. Griffith and Muhammad that is moving xenotransplantation into the clinical arena.”
Dr. Gallo added, “I want to congratulate my colleagues at the Department of Surgery, its leader, Dr. Christine Lau, and others who contributed to this successful transplantation. Further, in particular, I commend our Institute’s team of Drs. Saharia, Kottilil, and Amoroso and their colleagues, on their steadfast commitment to support this important program and their ongoing contribution in this unprecedented infectious disease control and detection program particularly in the face of an immune-suppressed challenging clinical environment.”
For more information: https://www.ihv.org/
Related content:
Pig Heart Transplant Patient Continues to Thrive
VIDEO: Details on the First Pig Heart Transplant Surgery in a Human
First Human Receives a Pig Heart Transplant
Transplanting Pig Hearts Into Humans One Step Closer
Read more on this heart transplant case: www.medschool.umaryland.edu/news/2022/University-of-Maryland-School-of-Medicine-Faculty-Scientists-and-Clinicians-Perform-Historic-First-Successful-Transplant-of-Porcine-Heart-into-Adult-Human-with-End-Stage-Heart-Disease.html
Related Heart Transplant News:
FDA OKs Use New Version of Carmat Artificial Heart in U.S. Early Feasibility Study
Carmat Artificial Heart Shows Promising Automatic Regulation of Blood Flow
Heart in a Box Technology Expands Heart Transplant Window
FDA Clears SynCardia 50cc Temporary Total Artificial Heart as Bridge to Transplant
Stephanie’s Heart: The Story of Baby Fae
Hearts from donors who used illicit drugs or overdosed safe for transplant, cuts wait time
National Trends in Heart Donor Utilization Rates: Are We Efficiently Transplanting More Hearts?
Heart transplants from donors with hepatitis C may be safe and could help decrease organ shortage


 February 03, 2026
February 03, 2026 









