May 15, 2019 — Renal artery denervation (RDN) was found to be safe when employed with pulmonary vein isolation (PVI) to patients with paroxysmal atrial fibrillation (AF) and hypertension and significantly increased the likelihood of freedom from AF in a single-blind, randomized clinical trial. Results from the ERADICATE-AF Trial were presented during a late-breaking session at Heart Rhythm 2019, the Heart Rhythm Society's 40th Annual Scientific Sessions.
Activation of the sympathetic nervous system plays an important role in the development and perpetuation of AFib. RDN can produce significant sympatholysis and a small randomized clinical trial has demonstrated enhanced efficacy when RDN is added to PVI for patients with AF and hypertension. The ERADICATE-AF Trial was designed to assess the safety and efficacy of RDN on long-term arrhythmic outcomes in a large single-blind longitudinal randomized clinical trial.
Patients with a history of hypertension (defined as SBP ≥ 130 and/or DBP ≥ 80 mmHg) despite ≥ one anti-hypertensive medication, and paroxysmal AF and plans for a guideline-supported catheter ablation, were eligible. Subjects were randomized 1:1 to PVI alone or PVI+RDN at five centers and were blinded to treatment assignment. Complete PVI was performed in all patients, and RDN by radiofrequency ablation catheters within each renal artery. The primary study endpoint was antiarrhythmic drug freedom from AF recurrence at 12 months (not including a three month blanking period). A sample size of 300 was calculated to provide 80% power to detect a relative 40% decrease in the one-year incidence of AF in patients treated with PVI+RDN compared to PVI alone.
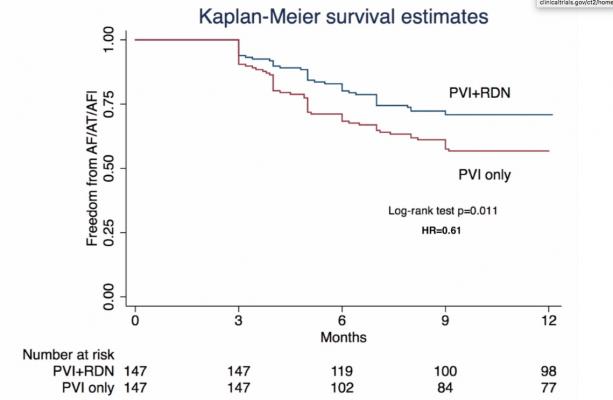
The target sample size of randomized patients was met in March 2019 — age 60.2 ± 6.4 years, 40% female; baseline BP 150.2±8.7/89.8±8.9 mm Hg. Patients were well matched between groups. Periprocedural complications occurred in 13 (4.4%), not different between groups; all resolved prior to discharge. At the end of 12 months, the PVI+RDN group had significantly greater freedom of AF than the PVI only group: 71.4 vs 57.8 percent (HR = 0.61 [95%CI = 0.41-0.90], p = 0.011, Figure).
Additional prespecified secondary endpoints, including blood pressure control, will be analyzed in the future. The study results showed consideration of adjunctive RDN is reasonable to increase the success rate of AF ablation in patients with HTN.
All the HRS 2019 late-breaking studies
Related Content:
VIDEO: New Approaches to Denervation Therapy
Renal Artery Denervation and Catheter Ablation Increased Freedom From Atrial Fibrillation
VIDEO: Device Therapies to Treat Hypertension
SPYRAL HTN-OFF MED Study Results Show Efficacy, Safety of Renal Denervation
Medtronic Announces Spyral HTN Global Clinical Trial Program for Renal Denervation
Medtronic Randomizes First Patients in Symplicity HTN-4
Medtronic Says its U.S. Renal Denervation Pivotal Trial Fails to Meet Primary Efficacy Endpoint
The Future of Renal Denervation Following the Failed SYMPLICITY HTN-3 Trial


 February 06, 2026
February 06, 2026 









