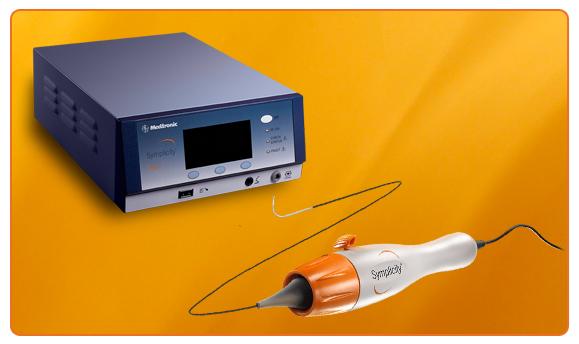
January 2, 2014 — Medtronic Inc. announced that the first patients were randomized in Symplicity HTN-4, which will evaluate the Symplicity
renal denervation system in patients with moderate uncontrolled
hypertension (systolic blood pressure greater than or equal to 140 and less than 160 mm Hg, despite treatment with three or more anti-hypertensive medications of different classes). Symplicity HTN-4, which randomized its first patients at Medical University of South Carolina (MUSC), Piedmont Heart Institute and Duke University Medical Center, builds upon Symplicity HTN-3, the only other renal denervation
clinical trial in the United States. In the United States, the Symplicity renal denervation system is available for investigational use only.
Symplicity HTN-4 will enroll up to 580 patients at approximately 100 sites, continuing to target a patient population in line with the Joint National Committee on the Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC-7), the American Heart Association (AHA) and the European Society of Hypertension's definition of uncontrolled hypertension. Similar to the U.S. trial, Symplicity HTN-3 study evaluating patients with uncontrolled hypertension with a systolic blood pressure greater than or equal to 160 mm Hg, the Symplicity HTN-4 study will be blinded and include a sham control.
Principal investigators of Symplicity HTN-4 are David Kandzari, M.D., director and chief scientific officer, Interventional Cardiology and Interventional Cardiology Research, Piedmont Heart Institute, Atlanta, and Michael Weber, M.D., professor of medicine, Division of Cardiovascular Medicine, SUNY Downstate College of Medicine, Brooklyn, New York City.
For more information: www.symplifybptrial.com, www.medtronicrdn.com