January 9, 2014 — Medtronic Inc. announced its U.S. pivotal trial for its Symplicity
renal denervation system to treat resistant hypertension, the SYMPLICITY HTN-3 trial, failed to meet its primary efficacy endpoint. The trial met its primary safety endpoint, and the trial's Data Safety Monitoring Board (DSMB) concluded that there were no safety concerns in the study.
This comes as a major blow to advocates for denervation therapy, which has been a hot new technology topic for interventional cardiology. Symplicity was the first renal denervation system to enter U.S. pivotal trials and was widely expected to be the first renal denervation system to be approved by the U.S. Food and Drug Administration (FDA). Several companies are now developing competing technologies renal denervation therapy technologies. However, the data from this trial now casts doubt on the Symplicity device and renal denervation therapy.
"SYMPLICITY HTN-3 met its primary safety endpoint related to the incidence of major adverse events one month following randomization and renal artery stenosis to six months," said Deepak L. Bhatt, M.D., M.P.H., executive director of interventional cardiovascular programs at Brigham and Women's Hospital Heart and Vascular Center, professor of medicine at Harvard Medical School and co-principal investigator of SYMPLICITY HTN-3. "Importantly, however, the trial did not meet its primary efficacy endpoint."
George Bakris, M.D., professor of medicine and director of the ASH Comprehensive Hypertension Center at the University of Chicago Medicine, past-president of the American Society of Hypertension and co-principal investigator of SYMPLICITY HTN-3 stated, "While it's disappointing the trial did not meet its primary efficacy endpoint, this is the most rigorous renal denervation clinical trial conducted to date, and the first of its kind to include a sham-control group. We look forward to advancing these data into the peer review process and will submit these findings for presentation and scientific discussion at an upcoming scientific congress."
Patient Safety
In light of the product's demonstrated safety profile, including the SYMPLICITY HTN-3 findings, no specific action is currently indicated for patients who have had the renal denervation procedure with the Symplicity system. Patients should consult with their physician regarding any questions they may have about their treatment.
Future of Medtronic’s Denervation Program
Based on these clinical trial findings, Medtronic intends to formulate a panel of independent advisors made up of physicians and researchers who will be asked to make recommendations about the future of the global hypertension clinical trial program, as well as provide advice on continued physician and patient access to the Symplicity technology in countries with regulatory approvals.
Pending this panel review, the company intends to:
- Suspend enrollment in the three countries where renal denervation hypertension trials are being conducted for regulatory approvals (SYMPLICITY HTN-4 in the U.S., HTN-Japan and HTN-India).
- Begin informing clinical trial sites and investigators, global regulatory bodies, and customers of these findings and decisions.
- Continue to ensure patient access to the Symplicity technology at the discretion of their physicians in markets where it is approved.
- Continue the Global SYMPLICITY post-market surveillance registry and renal denervation studies evaluating other non-hypertension indications.
"We are disappointed that the clinical trial failed to meet its primary efficacy endpoint," said Rick Kuntz, M.D., chief medical officer of Medtronic. "We believe this course of action is the most prudent and will help us thoroughly evaluate these findings and determine the appropriate next steps for renal denervation therapy."
Medtronic said the company is evaluating the carrying value of the renal denervation assets and based on the above trial results, believes a one-time impairment charge in the future will be likely. The company reiterated its previously communicated revenue outlook and diluted earnings per share guidance for fiscal year 2014.
SYMPLICITY HTN-3 Clinical Trial?
SYMPLICITY HTN-3 is the first U.S. blinded, randomized, controlled trial designed to evaluate the safety and effectiveness of renal denervation with the investigational Symplicity renal denervation system in patients with treatment-resistant hypertension and systolic blood pressure higher than 160 mmHg. Follow-up for all patients randomized in the trial will continue as planned out to five years.
The study randomized 535 treatment-resistant hypertension patients in 87 U.S. medical centers. People receiving the investigational treatment were compared with a sham-control group that did not receive treatment, with all patients continuing to take their blood pressure medications. Patients enrolled in the SYMPLICITY HTN-3 trial were randomly assigned to a group, with two out of three assigned to the treatment group and one out of three assigned to the sham-control group. In addition, those in the control group had the option to receive the treatment after the six-month assessment of the primary endpoint. The primary endpoints of the study are the change in office blood pressure from baseline to six months and incidence of major adverse events.
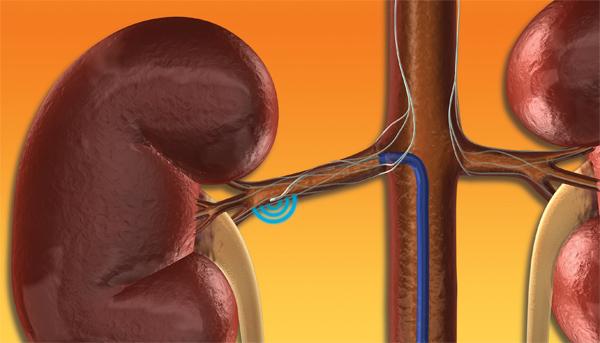
Symplicity Renal Denervation System?
The Symplicity renal denervation system use is similar to an angioplasty, where the physician inserts the small, flexible catheter into the femoral artery and threads it into both renal arteries in turn. Once the catheter tip is in place within the renal artery, the Symplicity generator is activated to deliver a controlled, low-power radio-frequency (RF) energy routine according to a proprietary algorithm aiming to deactivate the surrounding renal nerves. This, in turn, is intended to reduce hyper-activation of the sympathetic nervous system, which is an established contributor to chronic hypertension. The procedure does not involve a permanent implant.
For more information: www.medtronic.com, www.clinicaltrials.gov (Identifier: NCT01418261)


 November 14, 2025
November 14, 2025 









