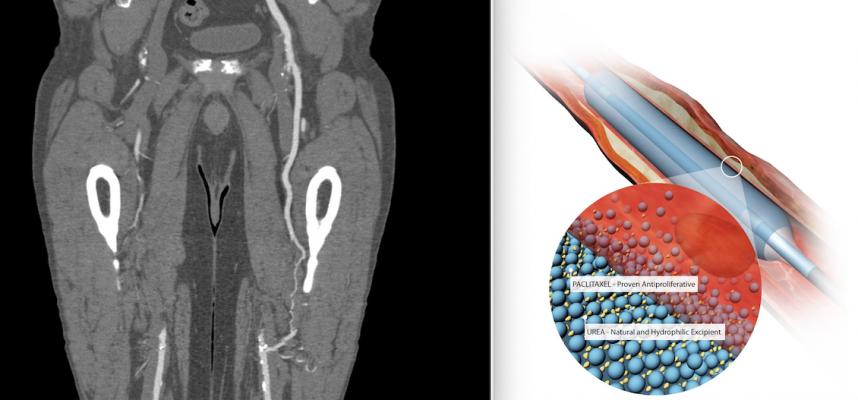
Two years ago a study raised concerns that paclitaxel-coated devices may cause increased mortality in peripheral artery disease patients. However, numerous studies now show there is no risk of increased mortality. The most recent study is the SAFE-PAD study presented at ACC.21.
June 7, 2021 — A couple years ago a study showed a mortality safety signal in patients who underwent peripheral artery disease (PAD) revascularization with paclitaxel drug-coated devices. This immediately raised alarm in with the U.S. Food and Drug Administration (FDA) and the interventional community and prompted numerous studies to confirm if there was a mortality signal, or if the findings were just coincidence. The latest study is SAFE-PAD, which showed drug-coated devices are noninferior to non-drug-coated devices for all-cause mortality in Medicare beneficiaries who had femoropopliteal revascularization.
The initial report from the SAFE-PAD study was presented May 16 in a Featured Clinical Research session during the ACC 2021 annual meeting, and simultaneously published in JAMA Internal Medicine.[1]
Eric A. Secemsky, M.D., MSc, FACC, et al., designed this retrospective cohort study with feedback from the U.S. Food and Drug Administration (FDA) to provide a longitudinal assessment of the safety of the drug-coated devices in this setting because of data from meta-analyses of small trials showing an association between drug-coated devices and increased mortality.
Among 168,553 Medicare fee-for-service beneficiaries (45% women, 82% white, mean age 77 years) who underwent femoropopliteal artery revascularization from 2015 through 2018, 51% had diabetes, 49% used tobacco, 46% had critical limb ischemia (CLI), and 8% had a previous amputation. A drug-coated device was used in 41.9%.
Results showed that after weighting, cumulative all-cause mortality over the median 2.72 years of follow-up (longest, 5.16 years) was 53.8% with drug-coated devices and 55.1% with non-drug-coated devices (hazard ratio, 0.95; 95% confidence interval, 0.94-0.97; noninferiority p<0.001). Sensitivity analyses were consistent and robust for the primary outcome.
This finding with drug-coated devices was consistent across prespecified subgroups, including treatment with stents or balloons, presence or absence of CLI, and the lowest quartile of total morbidities.
"SAFE-PAD will continue until the median follow-up of all patients surpasses 5 years and will provide the FDA a mechanism for the ongoing safety evaluation of these devices," write the authors. "Furthermore, SAFE-PAD may serve as a case example of how to leverage real-world evidence to provide timely device safety evaluations."
In a related editorial comment, Rita F. Redberg, M.D., MSc, FACC, and Mary M. McDermott, M.D., wrote "this analysis did not confirm the signal of harm seen in the [randomized clinical trials]." However, they explain that it raises "new and important questions" about the high rates of mortality in this population undergoing endovascular revascularization, and note that more than half of patients with [peripheral artery disease (PAD)] died during the 2.7 years of follow-up. Furthermore, patients with PAD have higher rates of death from cardiovascular events, and "recent evidence shows a high rate of death from cancer and infection" in PAD. Thus, there should be a continued focus on conservative treatment, including smoking cessation and exercise therapy to improve quality of life, they conclude.
Find more ACC 2021 late-breaking studies
Related Paclitaxel Safety Content:
No Increased Long-Term Mortality for the Paclitaxel-Coated Zilver PTX Stent
FDA Issues Letter About Paclitaxel Coated Balloons and Eluting Stents
Industry, Investigators Push Back With Patient-Level Data Showing No Increased Death From Paclitaxel-Based PAD Therapies
No Increased Long-Term Mortality for the Paclitaxel-Coated Zilver PTX Stent
FDA Issues Letter About Paclitaxel Coated Balloons and Eluting Stents
Top 10 Takeaways on Interventional Technologies at TCT 2020
No Mortality Risk Associated With Paclitaxel-coated Devices Used to Treat Peripheral Artery Disease
FDA Panel Recommends Continued Use of Paclitaxel-coated Peripheral Devices
Safety of Paclitaxel-eluting Stents and Balloons Called Into Question
6 Hot Topics in Interventional Cardiology at TCT 2019
The Data-driven Crises Involving Paclitaxel and Impella RP
VIDEO: SCAI Prospective on Key Takeaways at TCT 2019
Reference:


 July 31, 2024
July 31, 2024 









