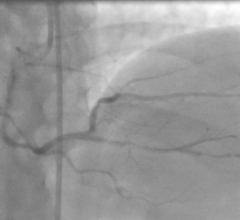
September 30, 2019 – The first randomized trial to compare a durable polymer drug-eluting stent to a polymer-free drug ...
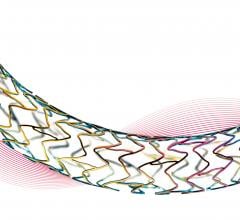
Stents
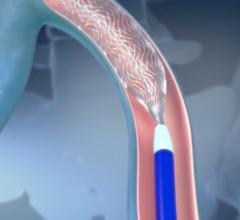
This channel includes news and new technology innovations for stents, also called vascular scaffolds. Stents are used to help prop open a vessel treated by balloon angioplasty because of the barotrauma caused by the extreme stretching of vessel walls. The stent enables to vessel to heal in an open position with collapsing. Drug eluting stents (DES) are coated in anti-proliferative drugs to precent scar tissue growth which can cause restenosis and occlude the vessel. DES require antiplatelet therapy because the drug carrier polymer on DES can cause thrombus inside the stent, even years after treatment, which is why bare metal stents are still used in some patients. This page includes news on coronary stents, carotid stents, peripheral stents, bioresorbable stents, and renal stents.
September 30, 2019 — A biodegradable polymer everolimus-eluting stent (BP-EES) followed by four months of dual ...
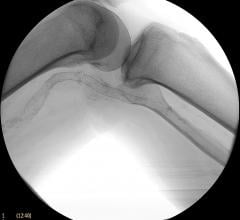
September 25, 2019 — Ten-year survival rates are similar for bypass surgery and coronary stenting with drug-eluting ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...

Primary percutaneous coronary intervention (PCI) is the preferred treatment for acute ST-segment elevation myocardial ...
September 5, 2019 — Biotronik's ultrathin Orsiro stent demonstrated superiority over Xience with respect to target ...

August 28, 2019 — The U.S. Food and Drug Administration (FDA) released an updated MedWatch Alert this month on the ...
August 21, 2019 — Increasing incidence of cardiovascular diseases worldwide is creating growth opportunities for the ...
August 1, 2019 — Less-invasive procedures to open severely clogged leg arteries were as good at helping people survive ...
June 13, 2019 — Silk Road Medical Inc. announced the presentation of real-world data for the treatment of patients with ...
May 6, 2019 — The U.S Food and Drug Administration (FDA) has cleared the Boston Scientific Vici Venous Stent System for ...
April 18, 2019 — The U.S. Food and Drug Administration published a draft guidance titled, “Technical Considerations for ...
April 15, 2019 – Intact Vascular Inc. received U.S. Food and Drug Administration (FDA) market clearance for the Tack ...
April 15, 2019 — Biotronik began its U.S. commercial launch of the PK Papyrus covered coronary stent system for use in ...
April 3, 2019 — The U.S. Food and Drug Administration (FDA) recently granted an additional indication to Bard Peripheral ...
April 3, 2019 — The U.S. Food and Drug Administration (FDA) recently cleared Bard Peripheral Vascular's Venovo Venous ...


 September 30, 2019
September 30, 2019