November 1, 2022 — Deepak L. Bhatt, MD, MPH, a top expert in cardiovascular medicine and interventional cardiology, has ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
November 1, 2022 — Abiomed announced that Impella RP Flex with SmartAssist has received U.S. Food and Drug ...
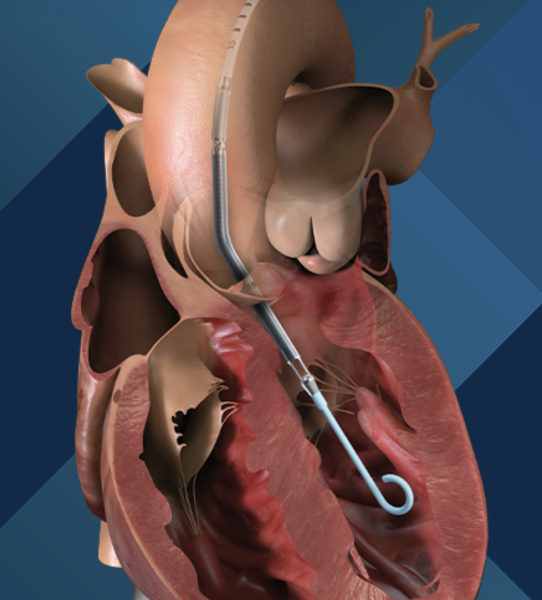
November 1, 2022 — Johnson & Johnson and Abiomed, a world leader in breakthrough heart, lung and kidney support ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
October 31, 2022 — Results from the Late-Breaking Clinical Trials session at The VEINS Conference in Las Vegas, NV, were ...
October 28, 2022 — V-Wave, announced today the completion of enrollment in RELIEVE-HF, a prospective, randomized ...
October 28, 2022 — SELUTION SLR, MedAlliance’s novel sirolimus-eluting balloon, has received FDA Investigational Device ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
October 27, 2022 — Vascular surgeons from Allegheny General Hospital (AGH) Cardiovascular Institute, the flagship ...
October 26, 2022 — Thousands of people have new hope for treatment of thoracic aortic arch disease and University ...
October 21, 2022 — Medtronic plc, a global leader in healthcare technology, today announced it has received U.S. Food ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
October 18, 2022 — HeartFlow, Inc., a leader in revolutionizing precision heart care, has received U.S. Food and Drug ...
October 17, 2022 — Allegheny Health Network’s (AHN) Cardiovascular Institute announced the expansion of its nationally ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
October 18, 2022 — Swiss-based medical technology company MedAlliance has announced it has entered into an agreement ...
October 11, 2022 — Efemoral Medical, developer of advanced interventional bioresorbable therapies, announced that it ...


 November 01, 2022
November 01, 2022