Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
June 12, 2023 — New research published in JMIR Cardio reveals the remarkable potential of artificial intelligence (AI) ...
Preventing contrast induced nephropathy and managing patients with chronic kidney disease remain major challenges in the ...
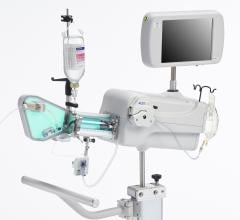
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
May 30, 2023 — Twelve-month results from the SELUTION SFA trial have been presented for the first time at the Japan ...
May 30, 2023 — The US FDA, on the 24th of May 2023, granted an Investigational Device Exemption (IDE) approval for Conce ...
Behavioral science is being applied in unique ways for enterprise imaging product design. Involving clinicians in the ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
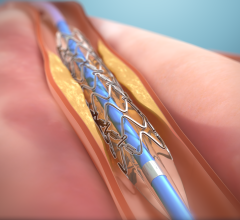
May 25, 2023 — First-generation bioresorbable vascular scaffolds (BVS) may be just as effective as drug-eluting metallic ...
May 25, 2023 — Boston Scientific Corporation announced data supporting use of the company's key electrophysiology and ...
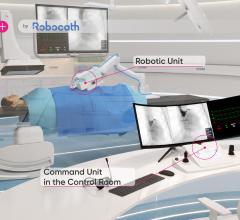
May 17, 2023 — Robocath, a company that designs, develops and commercializes smart robotic solutions for the treatment ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
May 17, 2023 — Edwards Lifesciences announced that new data from the Benchmark Registry in Europe demonstrated the ...
May 17, 2023 — Elixir Medical has reported that important data were presented at a late-breaking clinical session during ...

The Heart Rhythm Society (HRS) announced the full lineup of speakers and sessions, including late-breaking clinical ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
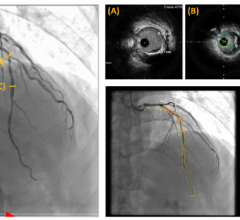
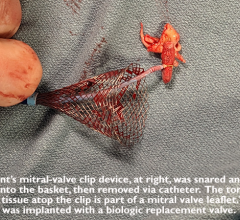
March 16, 2023 — Cardiologists at the UW Medicine Heart Institute recently performed a first-in-the-world procedure ...
May 16, 2023 — Henry Ford Health Interventional cardiologists William O’Neill, M.D., and Khaldoon Alaswad, M.D., took a ...
May 16, 2023 — Royal Philips, a global leader in health technology, announced late-breaking data from the DCR4Contrast ...

 June 13, 2023
June 13, 2023