February 15, 2023 — Abbott and Cardiovascular Systems, Inc. (CSI), announced a definitive agreement for Abbott to ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
February 14, 2023 — Biosense Webster, Inc., a global leader in cardiac arrhythmia treatment and part of Johnson & ...
February 2, 2023 — Abbott today announced two approvals as part of its growing suite of electrophysiology products in ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
February 2, 2023 — Technology is continuously evolving, and so is the approach taken by transplant cardiologists at Memo ...

Here is a recap of what DAIC viewers found most interesting during the month of January:
1. A Dietary Supplement ...
January 31, 2023 — Serotonin can impact the mitral valve of the heart and potentially accelerate a cardiac condition ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
January 27, 2023 — The first US patient has been enrolled at Medstar Washington Hospital Center in the SELUTION4ISR ...
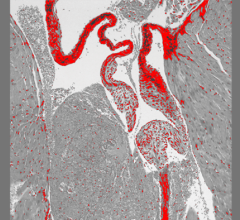
January 23, 2023 — A study of more than 100,000 patients has revealed that, for patients with blockages in multiple ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
January 23, 2023 — Imperative Care, Inc., announced U.S. Food and Drug Administration (FDA) 510(k) clearance of its Zoom ...
January 19, 2023 — Shockwave Medical, Inc., a pioneer in the development of Intravascular Lithotripsy (IVL) to treat ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
Behavioral science is being applied in unique ways for enterprise-imaging product design.
Behavioral science is the ...
January 12, 2023 — Selution SLR, MedAlliance’s novel sirolimus-eluting balloon, has received conditional FDA ...
January 12, 2023 — Medtronic announced the first patient enrollment in the ADVANCE Trial, a head-to-head randomized ...


 February 15, 2023
February 15, 2023