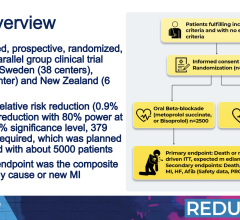
April 8, 2024 — Taking beta blockers after a heart attack did not significantly reduce the risk of death or a second heart attack among people with normal heart pumping ability, as indicated by an ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
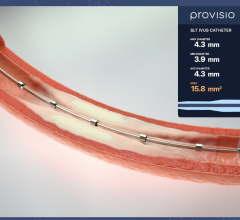
April 25, 2024 — Provisio Medical announced FDA 510(k) clearance of the Provisio SLT IVUS System. Sonic Lumen Tomography ...
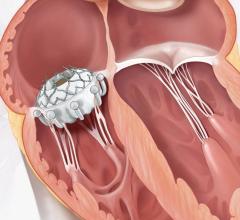
April 25, 2024 — Atlantic Health System’s Morristown Medical Center treated the first patient in New Jersey using Edward ...
April 24, 2024 — Expanse ICE announced today the ICE Aspiration System has received 510(k) clearance from the U.S. Food ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
April 17, 2024 — Getinge and Cook Medical announced an exclusive sales and distribution agreement for the iCast covered ...
April 16, 2024 — Each year more than 500,000 Americans undergo percutaneous coronary intervention, or PCI, a minimally ...
April 16, 2024 — Vivasure Medical, a company pioneering novel fully absorbable technology for percutaneous vessel ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
April 12, 2024 — Simpson Interventions, Inc., a pioneering medical technology company specializing in cardiovascular ...
April 11, 2024 — One-year success rates from angioplasty procedures to open clogged arteries in the legs were ...
April 8, 2024 — Taking beta blockers after a heart attack did not significantly reduce the risk of death or a second ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
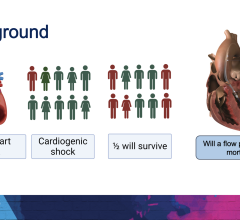
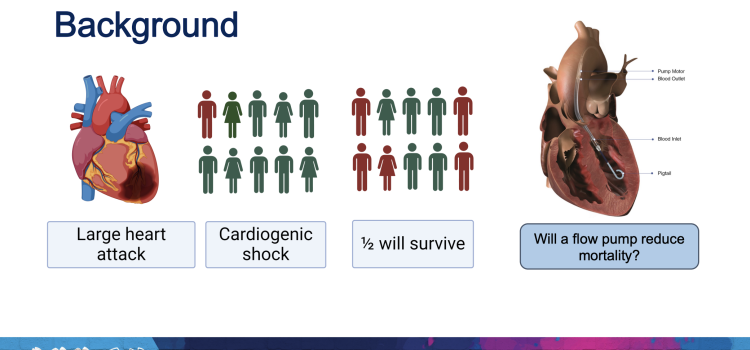
April 8, 2024 — Implantation of the Impella CP micro-axial flow pump in the hours after a heart attack significantly ...
April 8, 2024 — People who have had a heart attack or who are at risk for a heart attack and who stopped taking aspirin ...
April 4, 2024 — The U.S. Food and Drug Administration (FDA) announced that Medos International Sàrl is recalling Cerenov ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...

Here is a Top 10 look at the content that was trending during the month of March on dicardiology.com:
1. CLS Health ...
April 3, 2024 — The U.S. Food and Drug Administration (FDA) announced that Teleflex and Arrow International are ...
April 2, 2024 — Medical device technology developer Concept Medical has announced it has been granted Investigational ...



 April 25, 2024
April 25, 2024