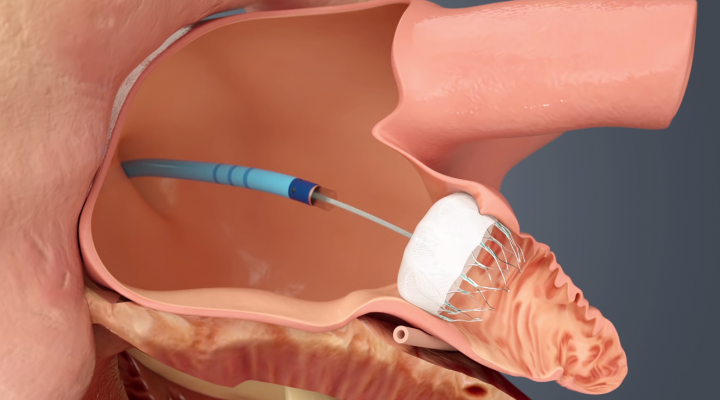
Patients with atrial fibrillation (AF or Afib) are high risk for stroke due to the formation of thrombus emboli in the left atrial appendage (LAA). The standard of care is use of warfarin or newer anticoagulation therapy agents, but there has been growing interest in eliminating the need for these drugs and their associated increased bleeding risks by closing off the LAA to prevent clot formation. Device therapy now exists for both open surgical and transcatheter LAA occlusion.
Below are links to recent DAIC articles that offer an update on this cardiovascular technology area:
Occluding the Left Atrial Appendage (LAA)
VIDEO - Post-FDA Approval Experience of LAA Occluders at ACC.16
FDA Approves Pivotal Trial for St, Jude's Amulet LAA Occluder
American College of Cardiology Launches Left Atrial Appendage Occlusion Registry
Societies Issue Recommendations for Left Atrial Appendage Occlusion
Biosense Webster Acquires Coherex Medical LAA Occluder Technology
Medicare Will Cover Watchman Left Atrial Appendage Closure Device
AtriCure Receives FDA Clearance for New AtriClip Device
New Study Demonstrates Cost-Effectiveness of Watchman Device
Boston Scientific Receives CE Mark For Next-generation Watchman FLX LAA Closure Device
FDA Announces Safety Issues With Lariat Left Atrial Appendage (LAA) Closure Device
SentreHeart Receives CE Mark for Lariat Suture Delivery Device
AtriCure Receives CE Mark for AtriClip PRO2 Device
New Data Supports St. Jude Medical FFR and LAA Closure Technology
AtriCure Announces First Patient Enrolled in FROST Cryoanalgesia Study
SentreHEART Receives FDA Approval for AMAZE Trial of Lariat Suture Delivery Device
Valley Hospital Begins Enrollment for aMAZE Trial
AtriCure Enrolls First Patient in ATLAS Study
Role of Interventional Echocardiography in Transcatheter Structural Heart Procedures


 August 28, 2023
August 28, 2023 









