March 8, 2010 – Bifurcation stent developer Tryton Medical Inc. recently announced its Tryton stent implants reached a milestone of 750 patients and recently had its first implants in Portugal and Austria.
“The Tryton Side Branch Stent is a simple approach to secure and dilate the side branch with optimal scaffolding of its ostium. Its use makes the placement and deployment of the main vessel stent easy,” said Francisco Pereira Machado, M.D., of Hospital da Luz in Lisbon, who performed the first implant in Portugal. “When there is involvement of both the main vessel and the side branch, and when, for any reason, you choose to go for two stents, think of dedicated stents. Keep it simple.”
Olev Luha, M.D., Ph.D., from the LKH University Hospital in Graz was the first to use the Tryton Side Branch Stent in Austria and has implanted four Tryton stents during four different procedures.
"I am pleased to have had the opportunity to start using the Tryton Side Branch Stent. The Tryton is easy to use and allows for a predictable treatment of bifurcation lesions, which are some of the most complex lesions that we get to treat. Going forward, I will use the Tryton Side Branch Stent in my daily practice," said Dr. Luha.
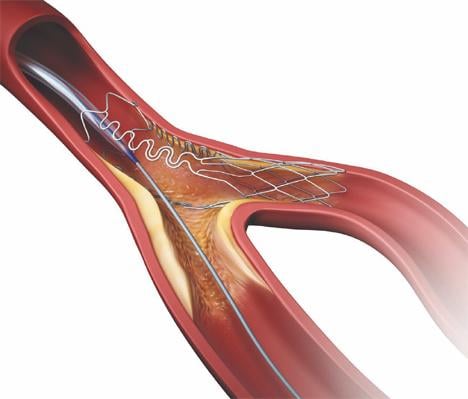
The Tryton Side Branch Stent System is designed to offer a dedicated strategy for treating atherosclerotic lesions in the side branch at the site of a bifurcation. These areas of the vascular system are a common location for plaque and are particularly challenging to treat with currently available stent systems. Approximately 22 percent of patients treated for coronary artery disease have diseased bifurcated lesions.
Tryton’s highly deliverable cobalt chromium stent is deployed in the side branch artery using a standard single-wire balloon-expandable stent delivery system. A conventional drug-eluting stent is then placed in the main vessel.
The Tryton Side Branch Stent System demonstrated excellent six-month clinical results in a first-in-man study of the system in 30 patients, with no restenosis occurring in the side branch artery. The stent system has received CE mark approval in Europe. It is not approved in the United States.
For more information: www.trytonmedical.com

