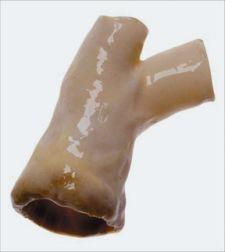
The FDA cleared CryoLife’s CryoValve SG pulmonary human heart valve processed with the company’s proprietary SynerGraft technology, providing a valve replacement option for children born with heart defects or for replacement surgery.
CryoLife’s proprietary SynerGraft technology is designed to remove allogeneic donor cells and cellular remnants from the valve without compromising the integrity of the underlying collagen matrix.
The CryoValve SG pulmonary human heart valve is indicated for the replacement of diseased, damaged, malformed or malfunctioning native pulmonary valves.

