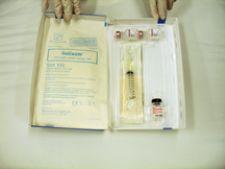
Baxter Healthcare Corp. received FDA approval in December for the GELFOAM Plus Hemostasis Kit (absorbable gelatin sponge, USP and human thrombin).
GELFOAM Plus is the only available hemostasis kit that contains Pfizer�s GELFOAM brand plus Baxter�s Thrombin (human) for use in controlling bleeding during surgical procedures.
Baxter and Pfizer signed a marketing, supply and manufacturing agreement for the GELFOAM Plus Hemostasis Kit. Under the terms of the agreement, Baxter is the exclusive distributor of the GELFOAM Plus Hemostasis Kit. Pfizer will provide the sterile GELFOAM gelatin absorbable sponge and Baxter will assemble, distribute and promote the GELFOAM Plus Hemostasis Kit.
Pfizer�s GELFOAM is indicated as a hemostatic device for surgical procedures when control of capillary, venous and arteriolar bleeding by pressure, ligature and conventional procedures are either ineffective or impractical.
February 2008

