March 23, 2009 - Pathway Medical Technologies Inc. today said the FDA granted the company 510(k) clearance to market the Jetstream G2 specifically for use in breaking apart and removing thrombus (or blood clots) from the upper and lower extremity peripheral arteries.
Jetstream G2, Pathway’s newest peripheral atherectomy catheter, is capable of treating the entire spectrum of disease found in patients suffering from peripheral arterial disease (PAD), including hard and soft plaque, calcium, fibrotic lesions and thrombus, with consistent results.
In the first six months on the market, the company said Jetstream was used to treat more than 600 patients at more than 100 centers. The company said the system offers a significant leap forward for atherectomy, and has enormous potential to revolutionize the PAD treatment market.
Approximately 12 million Americans suffer from the effects of PAD and many in this rapidly expanding patient population go undiagnosed. Commonly associated with high blood pressure, diabetes, heart disease, stroke and aging, PAD causes a build-up of plaque within the arteries that limits blood flow to the extremities. PAD can lead to severe limb pain, non-healing ulcers and critical limb ischemia and, if left untreated, can lead to gangrene, amputation and even death. The most common intervention for PAD has historically included highly-invasive procedures, including bypass surgery. Unfortunately, many patients are poor surgical candidates for whom surgery can be life threatening. Atherectomy, the removal of the blockage by inserting a specialized catheter into the artery to remove the fatty buildup from the artery walls, has proven to be an effective, first-line option in minimally invasive PAD intervention.
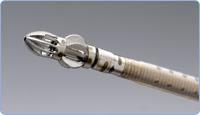
Jetstream G2, initially cleared by the FDA in January 2009, is a peripheral atherectomy catheter designed to remove all kinds of artery-clogging plaque in the lower limbs of patients. The Jetstream G2 consists of a sterile, single-use catheter and control pod and a reusable, compact console that mounts to a standard I.V. stand. The catheter has an expandable cutting tip that safely debulks and pre-emptively removes both hard and soft plaque, as well as calcium, thrombus and fibrotic lesions. A highly efficient aspiration port located just proximal to the cutting blades continually removes excised tissue and thrombus from the treatment site to a collection bag located on the console. A fully recessed masticating system within the aspiration port helps break aspirated material into smaller pieces before removal. The distal portion of the catheter also possesses infusion ports that provide continuous infusion of sterile saline during the atherectomy procedure. Active aspiration is a safety feature that minimizes the risk of distal embolization, the company said.
For more information: www.pathwaymedical.com

