The continuity within clinical conference topics from year to year and how presentations augment one another tells us a lot about where medicine is headed. Pulmonary embolism (PE) therapies were a key subject presented at the 2013 and 2014 Veith Symposium meetings in New York.
At the 2013 conference, attendees were introduced to the concept of the pulmonary embolism response team (PERT), as established at Massachusetts General Hospital. This multidisciplinary team — typically comprising practitioners in emergency medicine, critical care, pulmonology, cardiology, surgery and radiology — is summoned via a hot-line whenever a patient is diagnosed with PE, and its members collaborate to develop a streamlined, optimal plan of care. Together they review labs, imaging and clinical stats in real time and come to appropriate conclusions. This is much more efficient than the more traditional, often random approach of gathering input from specialists.
PERT, or similar collaborative teams, could and should become a conduit for specialists to share new techniques and advances, such as those gleaned from the Veith Symposium. Physicians sometimes tend to classify technology as disruptive when it is so significantly different that it alters how we normally handle patients or how we go about usual work.
The peripheral vascular sessions at last November’s symposium devoted attention to new technologies like the ultrasound-accelerated, catheter-directed thrombolysis (UACDT) in the treatment of pulmonary embolism. This technique’s application can be expected to grow, in light of the U.S. Food and Drug Administration’s (FDA) clearance last spring of the use of the EkoSonic Endovascular System for treating PE. It was the first FDA clearance for a new endovascular treatment option specifically for PE in more than a decade. For the approximately 530,000 patients in the U.S. each year presenting with symptomatic PE, this is a promising development.
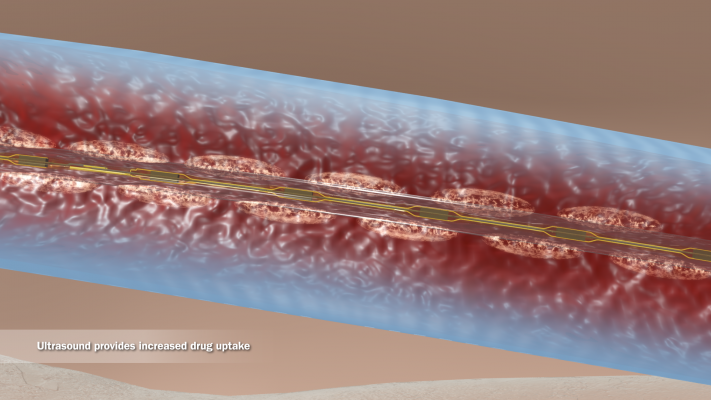
The application of ultrasound technology in thrombolysis creates microscopic pores in the clot, increasing clot permeability and penetration as well as exposure to plasminogen receptor sites. Acoustic streaming results in fibrin separation within the thrombosis and allows for active drug delivery. The tissue plasminogen activator (tPA) is forced deep into the clot and can act faster, with a reduced risk of hemorrhage. In short, physicians now have the potential to provide faster thrombolysis with a smaller tPA dose. At the University of Miami Hospital, we use the Ekosonic ultrasound-accelerated system to treat not only PE but deep vein thrombosis (DVT), peripheral artery thrombosis, visceral artery thrombosis, and renal or hepatic thrombosis. In these varied clinical situations, with the right patient-selection criteria, we have seen very good results.
While the majority of PE cases can be classified as minor PE and are highly treatable with oral anticoagulation therapy, evaluating cases of submassive PE and massive PE proves more challenging. Submassive PE has a mortality rate of 15 percent from progressive right ventricular failure and cardiovascular collapse; the risk of death rises to 50 percent in cases of massive PE. Survivors remain at risk for chronic thrombo-emboli and pulmonary hypertension.
Anticoagulation prevents the propagation of blood clots, but it can be ineffective when there is a large clot burden. Systemic thrombolysis carries a 20 percent risk of major hemorrhage and a 3-5 percent risk of intracranial hemorrhage. Partly due to these risks, in clinical practice thrombolysis is withheld in up to two-thirds of patients with massive PE.[1]
There is near-universal agreement that cases of massive PE warrant aggressive treatment such as ultrasound accelerated thrombolysis (USAT). Prospective and retrospective studies over the past 15 years have concluded that catheter-delivered thrombolysis is safe and effective and should be considered as first-line treatment for massive pulmonary embolism. Submassive cases were more open to debate about how to triage and stratify risks in applying the more aggressive thrombolysis.
Upon examining recent PE registries, patients with right ventricular dysfunction as identified by echocardiography usually faced more PE-related complications. For example, right ventricular strain doubled the risk of death within the first three months. After examining patients’ clinical presentations, we divided the submassive PE cases into two groups; those without right-heart strain received the more traditional treatment of IV heparin, while those with right-heart dysfunction were treated with USAT. Typically, using ultrasound-assisted, catheter-guided thrombolysis to administer a median tPA dose of 18 mg over 20 hours resulted in lowered right ventricular pressures (p<0.01), lower Mastora obstructive indices (p<0.01), and a median ICU stay of four days, with no major bleeding complications. To date, the University of Miami has treated more than 30 patients with similar results. This classification process has afforded good outcomes in our patients with submassive PE, establishing that ultrasound-assisted thrombolysis is safe and effective in reducing right ventricular strain.
In the 2014 ULTIMA Trial, 59 patients with submassive PE and evidence of right-ventricular dysfunction were randomized to receive either anticoagulation or ultrasound assisted catheter-directed thrombolysis. After 24 hours, the thrombolysis group realized a significant reduction in right and left ventricular end-diastolic diameter ratios, while there was no change in the anticoagulation group. Researchers concluded that ultrasound-assisted catheter-guided thrombolysis was superior to anticoagulation alone in reversing right ventricular dilatation without an increase in bleeding. The SEATTLE II trial was a larger, nonrandomized study that enrolled 150 patients (31 massive and 119 submassive PE) that also showed good efficacy for reducing patients’ right-heart strain within 48 hours after treatment with USAT. The results of these two trials were also related and discussed at other Veith symposium presentations.
If there were skeptics when USAT clinical experience was first presented in earlier years, the concept is becoming increasingly well received. While the literature supporting the use of ultrasound-accelerated, catheter-directed thrombolysis is growing, those in attendance at Veith seemed to agree on the need for well-controlled, large-scale clinical trials to establish the role of these treatments with evidence-based clinical guidelines. There is much interest in the results of the current ATTRACT trial studying catheter-directed thrombolysis in patients with DVT. Where previous trials have included 50-150 patients, the ATTRACT trial (an NIH-funded, Phase III, multi-center, randomized, controlled clinical trial) plans to enroll 692 patients. ATTRACT results are expected some time in 2015 and should offer data for review and discussion at the 2015 Veith Symposium.
Editor's note: Jason T. Salsamendi, M.D., is an assistant professor of clinical radiology at the University of Miami Hospital, and is certified in both diagnostic radiology and vascular and interventional radiology. He is involved in vascular and interventional oncology research, including use of the Ekos ultrasound accelerated thrombolysis system for PE.