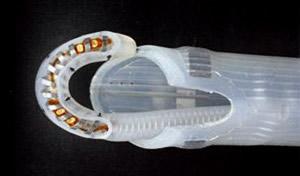
The EPi-Sense-AF Guided Coagulation System.
May 14, 2013 — nContact Inc. said it received conditional approval for an investigational device exemption (IDE) from the U.S. Food and Drug Administration (FDA) to begin enrollment in the CONVERGE trial, a multicenter, prospective, randomized study evaluating patients with symptomatic persistent atrial fibrillation (AF). AF is the most common cardiac arrhythmia, a condition that disrupts the ability of the atria (upper chambers of the heart) to beat regularly and pump blood efficiently. The CONVERGE study, designed to investigate the epicardial/endocardial Convergent Procedure, combines the cardiac ablation expertise, techniques, and technologies of both electrophysiologists and cardiothoracic surgeons.
"The Convergent Procedure may set a new standard of care in AF ablation," said Kenneth C. Civello Jr., M.D., MPH, of Our Lady of the Lake Hospital, Baton Rouge, La. "This pioneering clinical study will evaluate the unmet need of the large persistent AF patient population for whom conventional endocardial ablation has been limited."
The multidisciplinary Convergent Procedure is performed as a single procedure in the electrophysiology lab. The epicardial component of the procedure is completed with no chest incisions, using the proprietary transdiaphragmatic approach that requires a 2 cm incision in the abdomen, providing the surgeon direct visualization to create lesions across the atrium.
"The convergent procedure is the least invasive option among multidisciplinary ablation techniques; there is no chest violation, no heart dissection, and no lung deflation," said Peter Walts, M.D., of St. Vincent Medical Group, Indianapolis, Ind. "By providing direct visualization and closed-chest access to the posterior left atrium, the convergent procedure may address major limitations experienced in traditional endocardial ablation."
The CONVERGE trial will randomize patients 2:1 between nContact's epicardial/endocardial convergent procedure using the EPi-Sense-AF
Guided Coagulation System with VisiTrax and standalone endocardial ablation using fluid-irrigated catheters for the treatment of persistent AF. The primary effectiveness endpoint is freedom from AF, atrial tachycardia, and atrial flutter without the use of new antiarrhythmic drugs. Secondary effectiveness endpoints include the reduction of AF burden and changes in Quality of Life measures from baseline: patients will be followed for 12 months post-procedure. Primary and secondary safety endpoints are the incidence of major adverse events following the initial procedure and through the 12-month follow-up period.
For more information: www.ncontactinc.com


 February 06, 2026
February 06, 2026 









