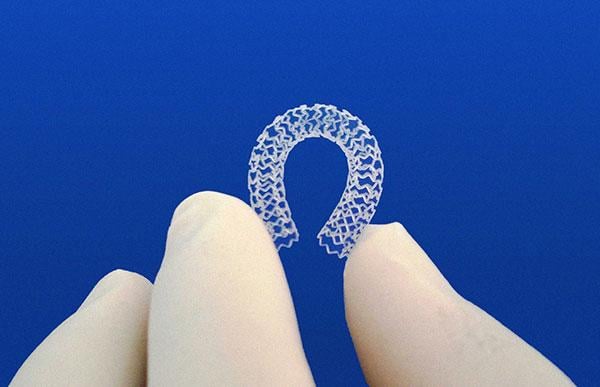
The Amaranth Fortitute bioresorbable stent was among several fully bioresorbing platforms discussed in sessions, late breaking trial data presentations and on the expo floor at TCT 2015.
There were several overarching technology trends seen at the 2015 Transcatheter Cardiovascular Therapeutics (TCT) annual meeting in San Francisco Oct. 11-15. The key technologies emphasized this year included new transcatheter devices in development to treat not only the aortic valve, but also the mitral and tricuspid valves. Bioresorbable stents and bioresorbable drug carrier polymers for metallic stents were heavily highlighted in sessions and on the show floor. Several TCT sessions also centered on the new economic realities hospitals face with pressures of healthcare reform.
Watch the video "New Technology Trends at TCT 2015," an interview with Tom Watson, clinical analyst for MDBuyLine, on key technology trends at TCT 2015.
Catheter-based Valve Replacement Continues to Grow
Catheter-based valve replacement continues to grow as advances in the design and available sizes of catheters improve and competing devices in the market increase. Clinical evidence also continues to support the adoption and success of transcatheter aortic valve replacement (TAVR). Clinicians and highly educated patients will likely be in favor of the FDA’s expanding the criterion that determines patient’s eligibility for the catheter-based approach. We expect this change will lead to a transition where catheter-based approaches are offered to a broader group of eligible patients, not only the sickest patients with little or no option for traditional surgery. Although we are not there yet, clinical experts believe this will be the future direction for catheter-based valve replacement.
An associated trend is the shift toward the transfemoral catheter approach over the transapical approach. The transapical approach is less invasive than open surgery, but it is still considerably more invasive than the transfemoral approach. Although some patients will still require the transapical method, we expect to see transfemoral become the more common approach.
Intersection of Hospital Efficiency, Economics and Clinical Outcomes
In today’s environment, hospitals continue to face pressure to streamline workflow, reduce costs and provide high-quality care in a cost-effective and efficient manner. This creates challenges when using best practices, leading to the use of more resources and time. For example, when determining appropriate patients for TAVR, physicians must use a multidisciplinary team to evaluate and determine those patients who are the best fit for the procedure. This creates a costly process in terms of resources and time between the evaluation and procedure. In addition, the procedure requires the involvement of a team of both interventional and surgical staff to ensure the best possible care for the patient. As TAVR continues to move more toward an interventional procedure and less often a surgical solution, when and how to use and or not use this multidisciplinary team approach will create challenges for hospitals.
TAVR is just one example of this issue all healthcare systems are currently facing or will face in the future. In many cases this means physicians and healthcare systems must evaluate upfront the effect of new and emerging technologies, sometimes without having all the clinical data they want. It is no longer acceptable to adopt the newest offerings without a deeper understanding of how the technology will affect workflow, cost and patient outcome, but there will be instances when higher-cost, new technology solutions and a less-than-complete evidence-based outcome will be at odds in decisions hospital medical and administrative directors face.
The Issue of Cost vs. Quality in Cath Labs
The dilemma of cost vs. quality is a common issue every facility faces. There will always be a need to provide high-quality patient care, but in a world of shrinking reimbursements and higher technology costs, this is becoming more difficult. However, the business of medicine cannot and should not take a backseat to quality, including in the cath lab.
At the Cath Lab Boot Camp at TCT, speakers highlighted how quickly or even if some cath labs will adopt new technology depends on whether there is a clear and measurable benefit to patient outcomes. However, even then, there will be more focus on the cost of this new technology vs. the benefit to patient outcome, at least initially.
Key areas that will become focal points include:
• Same-day discharge – not only for routine cases, but also for some of the more traditional complex cases.
• Reimbursement for same-day discharge has increased.
• Focus on optimizing the workflow but with an eye on all aspects of procedure and information documentation to ensure maximum reimbursement.
• Weighing the cost-benefit of in-procedure medication, such as anticoagulants, to provide quality care and outcomes but not at the expense of maintaining an acceptable profit margin.
However, these pressures and changes cannot be made in a vacuum. Educated and thoughtful decisions require proven clinical data. The consumer awareness of this technology also requires patient education on the pre- and post-procedure issues that can arise.
Update on Bioresorbable Stents (BRS)
The TCT offered several late-breaking trials to update the ongoing clinical studies related to comparison of BRS stents to the established drug-eluting stents (DES) on metal scaffolding:
• ABSORB III – everolimus-eluting bioreabsorbable scaffold vs. everolimus-eluting metallic stent in coronary artery disease (CAD) patients (ABSORB III Clinical Trial).
• ABSORB China – everolimus-eluting bioresorbable scaffold vs. everolimus-eluting metallic stent in CAD patients (ABSORB China Clinical Trial).
There were also first-report investigations for two trials:
• ABSORB II – everolimus-eluting bioresorbable scaffold vs. everolimus-eluting metallic stent in CAD patients – two-year outcomes (ABSORB II Clinical Trial).
• BIOSOLVE II – sirolimus-eluting bioresorbable metallic scaffold – six-month clinical, angiographic and imaging outcomes (BIOSOLVE II Clinical Trial).
These studies are of major interest to the cardiology community because the use of DES metal stents has become the standard of care for many percutaneous cardiac interventions (PCI) to open and maintain patency of lesions in the coronary arteries. However, metal stents are permanent, and there is continued concern for re-occlusion years after the stent is placed.
A bioresorbable device would allow delivery of a “short-term” mechanical support scaffold while drug-eluting capabilities would provide the acute and near-term treatment of these lesions but with the added benefit of a scaffold that would reabsorb within 24-36 months. This could conceivably reduce the chances of later occlusion. As a result, these clinical trials have been designed to prove noninferiority with the established DES metal stent.
In all of the ABSORB trials, the statistical data supports noninferiority. However, there is consensus that these trials have been underpowered with too few patients to be definitive. Additionally, there is some debate about the difference in statistically proven noninferiority and real-data proof.
Ultimately, because the basis of this device is improved long-term outcomes, we may not have an answer until we see results over five years or longer. Another consideration is this technology is projected to be considerably more expensive than the current use of DES on metal scaffolding. The dilemma then becomes a cost-risk benefit for hospitals as to whether, once FDA approved, to go with the higher cost BRS stents, but without the long-term data to prove associated evidence-based positive outcomes.
The BIOSOLVE II trial compares the first-generation bioresorbable “plastic” polymer scaffolding with a bioresorbable metallic scaffold that will reabsorb within 12 months. Data is immature at this point at only six months, and it is limited in terms of patients, as well.
The question will be, is there benefit or compromise to a scaffold that is reabsorbed in 12 months vs. 24-36? The same challenge with the ABSORB data remains. At what point are you able to determine the long-term benefit or comparison to the established DES on metal scaffolding that are the current standard of care? What will be the cost of the new devices? What will be the cost-benefit ratio to the patient outcomes? Only time will tell.
Other technology videos from TCT 2015:
Editor's Choice of the Most Innovative New Technologies at TCT 2015
Key Bioresorbable Stent Technology Presented at TCT 2015 — Dean Kereiakes, M.D., medical director of The Christ Hospital Heart and Vascular Center, and a lead investigator for both the ABSORB and SYNERGY stent trials, discussed the latest developments in bioresorbable stent technology.
New Technology Trends at TCT 2015 — Tom Watson, clinical analyst for MDBuyLine, and DAIC Dave Fornell discuss some of the technology trends at the 2015 Transcatheter Cardiovascular Therapeutics (TCT) annual meeting.
New Approaches to Denervation Therapy — Robert Schwartz, M.D., medical director at the Minnesota Heart Institute, explains new applications for denervation therapies for hypertension, heart failure and other disease.
Advances in Interventional Heart Failure Hemodynamic Support at TCT 2015 — Manesh Patel, M.D., associate professor of medicine, and director of interventional cardiology and the cath labs at Duke University, explains the innovations in interventional heart failure technologies, including the Aortix percutaneous heart pump.
Eye-tracking For Dose Reduction in the Cath Lab - Guillaume Baillaird, CEO of ControlRad Systems, described how his company's technology can significantly reduce radiation dose to staff and patients during angiography procedures.
Update on U.S. Transradial Access Adoption With Sunil Rao at TCT 2015
Advances in Intravascular Imaging at TCT 2015 — Ziad Ali, M.D., associate director of translational medicine, Columbia University Medical Center / New York-Presbyterian Hospital, explains recent advances in IVUS, OCT and FFR.
Lithoplasty as an Alternative to Treat Calcified Lesions — Todd Brinton, M.D., clinical associate professor and consulting associate professor of bioengineering at Stanford University Medical Center, explains balloon-based lithoplasty as a new alternative for treating heavily calcified coronary and peripheral vascular disease lesions.
Drug-Filled Stents as an Alternative to Polymer Drug Carriers — Dr. Stephen Worthley, Ph.D., director of the cardiac cath labs at Royal Adelaide Hospital at the University of Adelaide in Australia, and co-principal investigator of Medtronic's recently launched international drug-filled stent (DFS) clinical trial, explains DFS the new technology.
Editor’s note: Tom Watson joined MD Buyline in 1986 with over 35 years of experience in the field of cardiovascular medicine. At MD Buyline, Watson is responsible for the area of cardiology, cardiac cath and electrophysiology imaging and monitoring, cardiology PACS and cardiology information systems and a number of other areas. He graduated with a Bachelor of Science degree from Louisiana State University. He received registries in both noninvasive and invasive cardiovascular modalities through the National Society of Cardiopulmonary Technologies as well as adult echocardiography through the American Registry of Diagnostic Medical Sonographers.


 November 14, 2025
November 14, 2025 









