
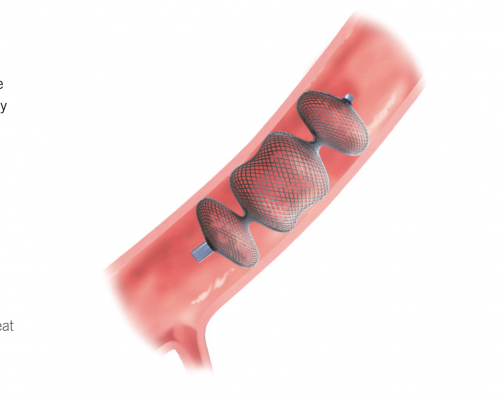
Transcatheter repair of a surgical mitral replacement paravalvular leak with an Amplatzer Vascular Plug II.
Off-label use of the St. Jude Amplatzer vascular plug devices offers a new solution for the minimally invasive repair of paravalvular leaks. Paravalvular leaks around surgical valve implants can result in poor outcomes, and the heart needing to work harder to pump can lead to heart failure. The small holes that cause paravalvular leak also form a Venturi effect, causing fast-moving jets that can lead to red blood cell destruction (hemolysis), causing anemia and fatigue.
Paravalvular leaks are a common issue with mechanical valve implantation. In 15-50 percent of cases, small paravalvular leaks are detected, but these are typically of minimal clinical significance.[1] However, in 1-5 percent of these cases, the leaks are associated with hemolysis, arrhythmias or congestive heart failure.[1-7]
Transcatheter repair of these leaks represents a new treatment option for these patients, who are often too frail for repeat surgery. Sessions on this technique presented at the Cardiovascular Research Foundation’s Transcatheter Valve Technologies (TVT) and Transcatheter Cardiovascular Therapeutics (TCT) conferences the past couple years have been packed. One of the key experts in this field is Charanjit Rihal, M.D., chief of cardiology and professor of medicine, Mayo Clinic, Rochester, Minn.
“The vast majority of our practice is with surgical valves, with 80 percent of these being mitral valves. And it seems to be a more common problem with mechanical valves than with tissue valves,” Rihal said.
He said the next most common repairs they perform are for surgical aortic valve replacements, followed by transcatheter aortic valve replacements (TAVR). Earlier versions of the Edwards Sapien and Medtronic CoreValve TAVR valves had paravalvular regurgitation, but the newest versions approved in 2015, especially the Sapien 3, have helped reduce this issue.
TAVR trials have shown an incidence of 10-20 percent of patients with paravalvular leak, and the data show this can lead to less than optimal outcomes, Rihal said. “In the best of all possible worlds there would be no paravalvular regurgitation, and we are optimistic that the next generation of TAVR valves will help eliminate this,” he said.
To seal these leaks, Amplatzer Vascular Plug II devices are used to fill the holes that have formed on the sides of the valve implant.
He said the devices do endothelize, but may not completely stop the leak, However, he said that is alright. “To treat the heart failure, we don’t have to eliminate the leak, we just have to reduce it. If we can reduce the regurgitation from severe to mild, the patient will feel much better. It is different for hemolysis, where it is very important to eliminate the leak, because even small, high-velocity jets can result in hemolysis,” he explained.
Friable Cardiac Tissue is Cause of Most Leaks
The high rate of leak with mitral valves is usually due to the patient’s valve annulus tissue being calcified. Rihal said surgeons have issues suturing in calcified tissue and the sutures may pull out with normal heart movement.
Other challenges Rihal’s team has found are with valve implants in post-endocarditis patients or the very elderly where the tissue tends to be very friable. This is particularly true if they have some form of chronic inflation problem, such as lupus or rheumatoid arthritis. He explained the tissue friability issue worsens if the patient is put on steroids. The condition causes sutures to just tear through the weakened cardiac tissue.
“You have to understand this is underdiagnosed,” Rihal said. “This is because it is often very difficult to visualize these leaks because on transthoracic echo [TTE] there is acoustic shadowing from the valve, the sewing ring and the calcium. So unless you are doing systematic transesophageal echo [TEE] surveys, which we usually don’t do, it is difficult to really know what the true incidence of this is.” Overall, he said the incidence is likely 5-10 percent of surgical valve replacements.
Imaging for the Procedure
Acoustic shadowing from TTE may totally obscure the view of some leaks, said Rebecca Hahn, M.D., director of interventional echocardiography, Columbia University Medical Center / New York-Presbyterian Hospital. Hahn spoke on the imaging needs for interventional paravalvular leak repairs at TVT 2015. For this reason, she said these procedures take place mainly under real-time 3-D/4-D TEE. Hahn said echo can confirm the delivery catheter is in the leak defect and not through the valve. It also can confirm the closure device is not touching the valve leaflets. Using color Doppler, echo can show the paravalvular jets to aid device navigation and help decide if additional devices are needed. It is also used for post-procedural assessments.
“The beauty of 3-D echo is that it allows us to also characterize the jets,” Hahn explained. “This allows us to pre-procedurally plan the devices we will choose, the sizing of the devices and the approach for deployment [transseptal or transapical].”
She said procedural navigation systems that merge live TEE with live angiography are not necessary, but are a great aid for novice operators in this type of procedure. For those operators, Hahn said these systems can significantly shorten procedural time.
The Procedure
“With the mitral valve, we have adopted an antegrade transseptal approach,” Rihal said. “You put a catheter into the left atrium and that is deflectable, so you can bend and steer it. Using that catheter, you pass a wire through the defect and then over the wire you send in a delivery guide catheter — it can be a coronary guiding catheter for example. Then, through that, there are a variety of plugs we can use.”
He said Mayo prefers to use thinner braid nitinol devices, such as the Amplatzer family of plugs, with the Amplatzer Vascular Plug II device being the workhorse for this kind of work. He said it is often necessary to use more than one plug to seal the holes. A tug test is usually performed to ensure the device is stable before its final release.
“None of these devices have been designed for this type of work, but there are no purpose-built devices and that has been the issue,” Rihal explained. “So we are sort of jury-rigging these devices to treat this very important problem. For that reason, we often have to use two or three of these devices to completely seal it off since we don’t have a single purpose-built device to do the job. I think they are pretty effective, and about 99 percent of the time we can get adequate closure. We usually cannot completely eliminate the leak, but we can help improve the patient’s symptoms and relieve their hemolysis as well.”
While the Amplatzer vascular plug was originally designed and cleared for vascular embolization, Rihal explained the design makes it ideal for closing paravalvular leaks. It has two self-expanding nitinol wire disks that open on either side of the valve, similar to the Amplatzer septal defect occluders, but are made of a finer wire. The plugs also have a raised, self-expanding center section that Rihal said helps fill the “nooks and crannies” along the side of the valve. The center section of the device has six layers of nitinol mesh, which helps occlude the hole. The Amplatzer II comes in sizes of 3-22 mm.
He said the Amplatzer III also seems to be a good device for this application, but it is not available in the United States. That device is approved in Europe and cardiologists there are using it to close paravalvular mitral leaks. The Amplatzer III is more of an oval/rectangular shape instead of a circle, which Rihal said might make it a good candidate for paravalvular leak closure.
“The Amplatzer IV is an interesting device because it seems to be ideally suited for post-TAVR paravalvular leaks,” Rihal said. “It will fit into anything that will accommodate an 0.038 wire, including a diagnostic catheter, making the device more versatile.”
He said the Amplatzer I is not useful for leak closures.
For very large defects, larger sheaths are best to use because you can get multiple devices through them, said Samir Kapadia, M.D., professor of medicine, director, cardiac catheterization laboratories, Cleveland Clinic, who also presented at TVT 2015. He showed one case where he used a 20 French Cook sheath to facilitate deployment of what was originally planned to be three Amplatzer II devices at once. The defect was so large that it required the use of eight devices. He said it is easier to use a large sheath and use vascular closure devices to seal the entry site than to use a smaller sheath when multiple devices will be needed.
Creating Dedicated Devices
The tearing of sutures usually causes irregular shaped holes. Rihal suggested if a new dedicated device were created, he would build a universal device that could be used to treat all patients. He explained it would need something like a liquid polymer injected into its core to seal the interior of the device once implanted to completely fill the hole and prevent any residual regurgitation.
In October 2014, the Occlutech Paravalvular Leak Closure Device (PLD) was the first dedicated paravalvular leak occluder device to gain European CE mark market clearance. The device looks like a square version of the Amplatzer, using a similar flexible, self-expanding nitinol wire mesh. The implant is available in different configurations, multiple sizes and can accommodate a broad range of paravalvular leak anatomies. The device can be delivered transapically or transfemorally. Placement is aided by two gold markers that can be visualized on angiography.
References:
1. Bhindi R, Bull S, Schrale RG, Wilson N, Ormerod OJ. “Surgery insight: percutaneous treatment of prosthetic paravalvular leaks.” Nat Clin Pract Cardiovasc Med 2008;5(3):140–7.
2. Pate GE, Al Zubaidi A, Chandavimol M, Thompson CR, Munt BI, Webb JG. “Percutaneous closure of prosthetic paravalvular leaks: case series and review.” Catheter Cardiovasc Interv 2006;68(4):528–33.
3. Webb JG, Pate GE, Munt BI. “Percutaneous closure of an aortic prosthetic paravalvular leak with an Amplatzer duct occluder.” Catheter Cardiovasc Interv 2005;65(1):69–72.
4. Sorajja P, Cabalka AK, Hagler DJ, Reeder GS, Chandrasekaran K, Cetta F, Rihal CS. “Successful percutaneous repair of perivalvular prosthetic regurgitation.” Catheter Cardiovasc Interv 2007;70(6):815–23.
5. Dussaillant GR, Romero L, Ramirez A, Sepulveda L. “Successful percutaneous closure of paraprosthetic aorto-right ventricular leak using the Amplatzer duct occluder.” Catheter Cardiovasc Interv 2006;67(6):976–80.
6. Echevarria JR, Bernal JM, Rabasa JM, Morales D, Revilla Y, Revuelta JM. “Reoperation for bioprosthetic valve dysfunction. A decade of clinical experience.” Eur J Cardiothorac Surg 1991; 5(10):523–7.
7. Zoghbi WA, Chambers JB, Dumesnil JG, Foster E, Gottdiener JS, Grayburn PA, et al. “Recommendations for evaluation of prosthetic valves with echocardiography and Doppler ultrasound: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Task Force on Prosthetic Valves.” J Am Soc Echocardiogr 2009;22(9):975–1014.
8. Kurt S. Hoffmayer, Christian Zellner, Damon M. Kwan, et al. “Closure of a Para-valvular Aortic Leak with the Use of Amplatzer Devices and Real-Time 2− and 3-Dimensional Transesophageal Echocardiography.” Tex Heart Inst J. 2011; 38(1): 81–84.







 November 14, 2025
November 14, 2025 









