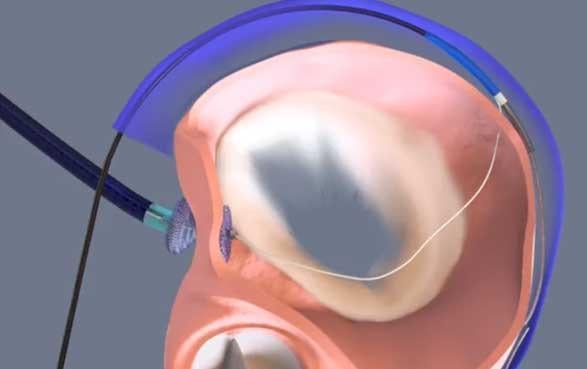
May 26, 2017 — Recent outcomes from the MAVERIC clinical trial confirm earlier positive findings that MVRx’s Arto System safely and effectively treats mitral valve regurgitation associated with congestive heart failure. The results, presented by leading cardiologists at EuroPCR 2017, May 16-19 in Paris, France, offer heart patients the promise of a less-invasive treatment for a life-threatening problem that affects more than 5 million people worldwide.
The ARTO System, a proprietary implantable device, is designed to improve heart failure symptoms by reducing mitral valve regurgitation, or the backward leakage of blood through the mitral valve, thereby increasing the forward flow of blood to the rest of the body. As a result, the heart functions more effectively and efficiently, leading to improved circulation, breathing and exercise tolerance.
The three-year Phase I portion of the MAVERIC study included 11 patients enrolled at Pauls Stradins University Clinical Hospital, Riga, Latvia. The results, presented by principal investigator Andrejs Erglis, M.D., Ph.D., demonstrate that the Arto System continues to provide a strong safety profile with 100 percent device success. Researchers found no instances of coronary artery compression, significantly reduced mitral valve regurgitation and significantly improved New York Heart Association (NYHA) heart function classification, a measure of heart failure. Moreover, heart-failure hospitalization fell 64 percent in the two years after Arto treatment compared with the two years prior. These results match those previously reported at earlier time points.
The current 30-day results from the multi-center MAVERIC trial were presented by Stephen Worthley, MB, BS, Ph.D., on behalf of the MAVERIC investigators. The report encompassed 45 patients inclusive of the 11 Phase I patients and represents a significant acceleration of enrollment beyond the single-center study. The 30-day results demonstrate that the Arto System is safe with 100 percent device success; there were no deaths, strokes or myocardial infarctions. At one month the findings also showed significantly reduced mitral valve regurgitation and significantly improved heart function. The 30-day results demonstrate that the safe and beneficial performance seen in the Phase I single-center study persists in a multi-center multi-operator setting.
For more information: www.mvrxinc.com


 November 14, 2025
November 14, 2025 









