November 18, 2013 — The first two commercial implants of the Boston Scientific Lotus
Valve System have taken place in a German hospital. Gerhard Schuler, M.D. and professor, University of Leipzig, Leipzig, Germany and Axel Linke M.D. and professor Leipzig, led the procedures at the Heart Center — University Hospital Leipzig. CE mark approval for the Lotus Valve System was announced Oct. 28 at the Transcatheter Cardiovascular Therapeutics conference (TCT 2013) in San Francisco.
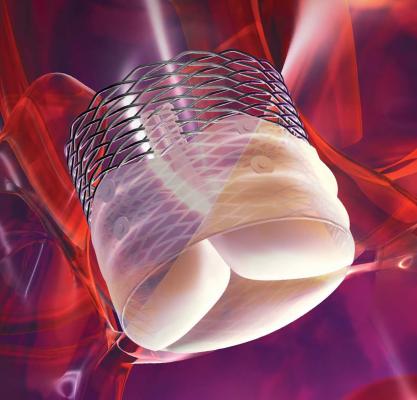
The Lotus Valve System offers an alternative treatment for patients with severe aortic stenosis at high risk of surgical valve replacement. It is a transcatheter aortic valve implantation (TAVI) device designed to give physicians total control throughout the TAVI procedure. The Lotus Valve is comprised of bovine pericardium and a nitinol frame with a central marker to aid in precise positioning. It features a novel Adaptive Seal technology to help minimize aortic regurgitation. It is also the first device of its kind that can be fully retrieved, redeployed or repositioned, even after full valve deployment and prior to release.
"The Lotus Valve System permits very precise positioning of the device and the Adaptive Seal minimizes potential paravalvular leakage," said Schuler. "These are the key differentiating features of this new technology."
Ian Meredith Ph.D., director, MonashHeart at Monash Medical Centre, Melbourne, Australia and principal investigator presented the primary endpoint data from the REPRISE II clinical trial at TCT 2013. The data demonstrated that the Lotus Valve System was successfully implanted and correctly positioned in all 120 patients, and met the co-primary endpoints of mean aortic valve pressure gradient and all-cause mortality at 30 days. The valve produced impressive clinical results with no valve malpositioning, migration or severe embolization, low clinical event rates that were consistent with those reported for other valves and negligible paravalvular aortic regurgitation at 30 days.
The Lotus Valve System comes pre-attached on a transfemoral delivery system and is inserted into the body through a small incision in the leg. Once delivered across the diseased aortic valve, the Lotus Valve System is deployed through a controlled mechanical expansion that is distinct from balloon-expandable or self-expanding valves.
"The controlled mechanical expansion and early functioning of the valve facilitate precise positioning on the first attempt, and the ability to fully or partially recapture the valve, if necessary, provides additional assurance that the valve will be ideally positioned at the end of the procedure," said Linke.
The Lotus Valve System is available at select centers in Europe with commercial site expansion accelerating as physicians and centers become fully trained. The valve is available in a 23 and 27 mm size, treating patients with aortic annulus sizes from 20 to 27 mm. The Lotus Valve System is an investigational device in the United States and Japan and is not available for sale.
"Completing our first commercial implants marks a key step forward in offering an advanced new technology in Europe,” said Tom Fleming, vice president and general manager, structural heart, Boston Scientific.” The Lotus Valve System has been designed to give the physician increased control during implantation and to help provide a more precise, predictable procedure. We believe the Lotus Valve is an important treatment alternative for severe aortic valve disease patients at high risk for surgical valve replacement."
For more information: www.bostonscientific.com