May 13, 2020 — The PRECEPT study testing the use of the Biosense Webster Thermocool Smarttouch SF Catheter for the treatment of persistent atrial fibrillation (AF), resulted in freedom from any documented, symptomatic atrial arrhythmias at 15 months post-procedure for eight out of ten study participants (80.4 percent).[1] The trial was presented as a late-breaking study at the Heart Rhythm Society (HRS) 2020 Science virtual meeting. Data from the PRECEPT study was also recently published in JACC: Clinical Electrophysiology.[1]
This PRECEPT study data will be used to support a premarket approval (PMA) supplement application to the U.S. Food and Drug Administration.
Atrial fibrillation (AF) is a significant public health issue affecting millions of people and placing a critical burden on healthcare systems. In the U.S., there are 5.5 million patients diagnosed with AF, and it's estimated to grow to more than 7 million by 2035.[2]
"Catheter ablation therapy is a widely accepted treatment option for atrial fibrillation, and the success in paroxysmal atrial fibrillation has been well established," said Moussa Mansour, M.D., associate professor in medicine at Harvard Medical School, director of both the cardiac electrophysiology laboratory and the atrial fibrillation programat Massachusetts General Hospital, and study investigator. "This data is encouraging and demonstrates that patient-tailored, radiofrequency catheter ablation therapy may provide a clinically meaningful benefit to patients with persistent atrial fibrillation, who are at an even higher risk for stroke and other complications."
Persistent AF is defined as continuous AF that lasts for more than seven days, while paroxysmal AF stops on its own or with intervention within seven days.[3] Approximately one-third of patients with paroxysmal AF will progress to persistent AF with 15 percent progressing within one year.[4,5] There is an increased physical and economic burden resulting from persistent AF, and it is associated with a higher risk of stroke, heart failure, and mortality.[6] The management of persistent AF aims to prevent AF recurrence and associated disabilities while reducing side effects from treatment.
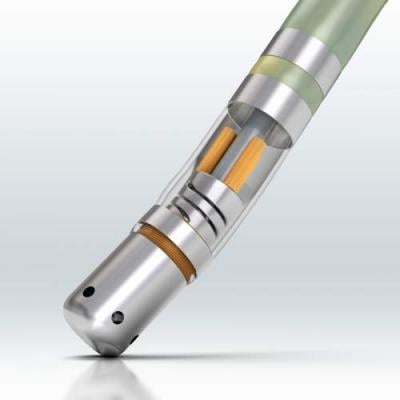
The PRECEPT study is the first prospective, multicenter investigational device exemption study designed to evaluate the safety and effectiveness of radiofrequency catheter ablation in patients with persistent AF, and was conducted using the Thermocool Smarttouch SF Catheter. The primary effectiveness endpoint was freedom from documented recurrence of atrial flutter/atrial tachycardia episodes of 30 seconds or longer and freedom from additional five failure modes: acute procedural failure, use of non-study catheter, repeat procedures, use of new/higher dose antiarrhythmic drugs, surgical AF ablation. At 15 months post-procedure, 86.1 percent of patients had freedom from the need for repeat ablation, and 80.4 percent of patients remained free from any documented, symptomatic atrial arrythmias.[1]
About the PRECEPT Study
The PRECEPT study utilized the VISTAG Module included in the CARTO 3 System and Thermocool Smarttouch SF Catheter to conduct patient-specific tailored ablation strategies requiring the isolation of all pulmonary veins and allowed for additional left atrium ablations when needed. A total of 381 patients with drug-refractory symptomatic persistent AF were enrolled at 27 centers in the U.S. and Canada. All patients received pulmonary vein isolation of all veins, and 44.5 percent of patients also received additional left atrium ablations. Primary adverse events occurred in 3.8 percent of patients, a rate similar to those reported in paroxysmal atrial fibrillation studies.[7,8] The average procedure time was under three hours (178 minutes), with an average fluoroscopy time of 15.3 minutes.[1]
What is Atrial Fibrillation
Atrial fibrillation (AF) is the most common type of cardiac arrhythmia (abnormal heart rhythm) and affects nearly one percent of the population.[9] During AF, the upper chambers of the heart, the atria, beat rapidly or in an uncontrolled manner, which can feel like a flutter. When the heart beats erratically, it does not pump blood as efficiently as it should. When oxygen is not being properly delivered to all parts of the body, the patient may feel ill or experience other AF symptoms. Atrial fibrillation may not be life-threatening; however, it is important to seek treatment to control the symptoms, as AF can lead to stroke.
For more information: www.biosensewebster.com
Find links to all the Heart Rhythm Society 2020 Late-Breaking Clinical Trials in Electrophysiology
References:
9. Johan E.P. Waktare, MB, ChB, MRCP, Atrial Fibrillation, AHA Journals.org.


 February 06, 2026
February 06, 2026 









