May 15, 2024 —CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for cardiac arrythmias, announced its participation at the Heart Rhythm 2024 conference, taking place in person at the Boston Convention & Exhibition Center and virtually, May 16-19, 2024.
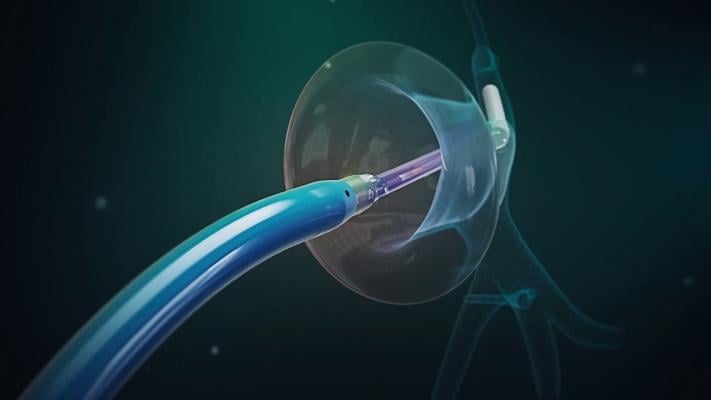
CardioFocus will feature its portfolio of innovative ablation systems which optimize lesion creation via confirmed tissue contact and sophisticated energy delivery. This will include the foundational HeartLight X3, the next generation ultra-compliant PFA balloon for true single shot pulmonary vein isolation, as well as the recently acquired Centauri™ Pulsed Electric Field System, and QuickShot™ large-area focal PFA catheter. Using a novel proprietary waveform, Centauri is compatible with several marketed focal ablation catheters and mapping systems.
"Our pursuit of innovation towards clinically meaningful ablation systems is driven by a focus on patient outcomes" said CardioFocus CEO Steve Ogilvie. "At Heart Rhythm 2024, we will present new data and products demonstrating our ability to customize proprietary pulsed field waveforms with each catheter system to deliver precise energy for safe and durable lesion creation."
CardioFocus will present new clinical data at two abstract sessions as well as a Rhythm Theater presentation titled, "Shaping The Future of Cardiac Ablation: How Contact and Waveform Science Enhance PFA's Lesion Durability".
Data Driven Pulsed Field Abstracts
Shephal K. Doshi, MD, FACC, Pacific Heart Institute, Santa Monica, CA, and Keita Watanabe, MD, Mount Sinai Hospital, New York, NY, will present data supporting Centauri and companion QuickShot catheter, and CardioFocus' pulsed field ablation balloon for true single shot pulmonary vein isolation, which is currently in development:
- PO-02-108 – Pulsed Field Ablation of Atrial Fibrillation Using a Large-Area Focal Catheter with a Novel Contact Detection Algorithm – Invasive Remapping Results for the QuickShot Catheter & CENTAURI System – Poster Session II – Ablation – Friday, May 17, 1:00-3:00p.m. EDT – Abstract Pavilion
- PO-06-144 – First Demonstration of a Multielectrode Endoscopic Balloon Pulsed Field Ablation Catheter: Initial Preclinical Feasibility – Poster Session VI - Ablation – Sunday, May 19, 9:30-11:30a.m. EDT – Abstract Pavilion
Rhythm Theatre Presentations on Pulsed Field Lesion Durability
Dr. Shephal K. Doshi will chair a Rhythm Theater presentation titled, "Shaping The Future of Cardiac Ablation: How Contact and Waveform Science Enhance PFA's Lesion Durability." Presentations will be made by Hiroshi Nakagawa, MD, PhD, Cleveland Clinic, Cleveland, OH, David Kenigsberg, MD, FHRS, FACC, Westside Regional Medical Center, Plantation, FL, Jacob Koruth, MD, Mount Sinai Hospital, New York, NY, and Jim Hansen, MD, DMSc, Gentofte Hospital, Hellerup, DK, on May 18, 2024, from 10:30a.m.-11:30a.m. EDT, in Rhythm Theater #2.
Exhibit Hall Booth and Tech Suites
CardioFocus' home at Heart Rhythm 2024 will be at Booth 653, where the company will release new visual branding to capture CardioFocus' foundation and future, combining both elements into a modern design. The booth will highlight HeartLight X3 and the Centauri PFA System. CardioFocus will also play host at two Tech Suites (TS254 and TS256), sharing products that the company is currently developing, including navigation enabled QuickShot and the true single shot ultra-compliant PFA balloon.
Abstracts will be available May 16, 2024. Abstracts and presentations can be accessed, once live, at: https://heartrhythm.com/.


 June 08, 2023
June 08, 2023 

