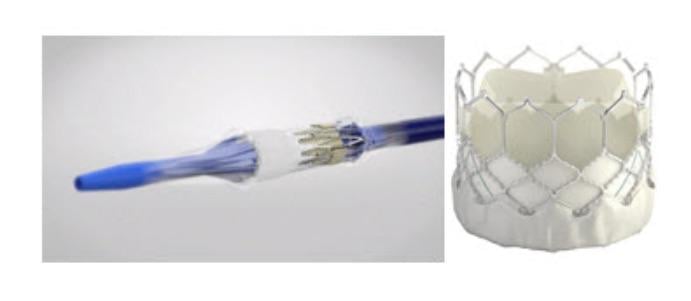
August 22, 2019 — Edwards Lifesciences is recalling the Sapien 3 Ultra delivery system after receiving reports of burst balloons during implantation procedures, which have resulted in significant difficulty retrieving the valve into the catheter and withdrawing the system from the patient. The company said this may cause vascular injury, bleeding or surgical intervention.
Seventeen injuries and one death were reported at the time when Edwards initiated the Field Corrective Action in July 2019.
The Edwards Sapien 3 Ultra delivery system is a part of the Edwards Sapien 3 transcatheter heart valve system. It is used to deliver and deploy the Edwards Sapien 3 Ultra or the Edwards Sapien 3 transcatheter heart valve to replace a diseased aortic valve without open-heart surgery. The Sapien 3 is indicated for use in patients with severe, symptomatic, aortic valve stenosis, a narrowing of the heart’s aortic valve that restricts blood flow to aorta, the body’s main artery.
The recall affects all lot numbers manufactured between Jan. 23, 2018 and July 16, 2019; the devices would have been involved in procedures starting Jan. 3 of this year. A total of 1,585 devices in the U.S. are affected by the recall.
In the Urgent Field Safety Notice to customers, Edwards provided the following recommendations and instructions for physicians:
-
When deploying the valve, inflate the balloon slowly and continuously throughout deployment. Hold for 3 seconds at full inflation. The delivery system requires a prescribed volume for THV deployment and proper function (11 mL, 17 mL, 23 mL, 33 mL).
-
The following warning will also be added to the Edwards Sapien 3 Ultra Transcatheter Heart Valve System Instructions for Use (IFU): "Failure to use slow, controlled inflation and prescribed nominal inflation volumes may result in balloon rupture, difficulty retrieving the delivery system and may require subsequent conversion to surgical intervention."
-
If a balloon burst is suspected, do not attempt to pull back the delivery system into the sheath until you are prepared to conduct the following technique:
-
Close stopcock to the delivery system and remove inflation device from stopcock.
-
Continuously twist the handle in a clockwise direction (full 360° rotations) while gently pulling back the delivery system into the sheath tip. Verify delivery system tip has entered the sheath tip under fluoroscopy.
-
Do not force if resistance is met near or at the sheath tip. Forcing retrieval when meeting resistance could result in additional balloon material tearing or tip dislodgement. Consider utilizing other interventional techniques for retrieval (e.g., a snare).
-
If successful in pulling the entire balloon into the tip of the sheath, withdraw the delivery system and sheath as a single unit completely from the arteriotomy while maintaining guidewire position. Do not attempt to pull the delivery system through the remaining length of the sheath.
-
If resistance is still encountered, convert to surgery for device removal. Based on medical assessment of the size, tortuosity and extent of calcification of the peripheral vessels, evaluate the risks and tradeoffs of carefully withdrawing the system into a more peripheral anatomy in order to allow a more localized procedure. Consider use of an occlusion balloon to mitigate bleeding risks.
-
Edwards also asked physicians to review the acknowledgment form, sign and date it, and return it to their Edwards representative or fax/email it as instructed on the form attached.
Healthcare professionals and consumers may report adverse reactions or quality problems they experience using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program either online, by regular mail or by Fax to 1-800-FDA-0178.
For more information: www.edwards.com


 November 14, 2025
November 14, 2025 









