February 22, 2010 – The FDA said Physio-Control Inc. has improved the quality of its external defibrillators and may resume unrestricted worldwide shipments.
In May 2008, Physio-Control signed a consent decree with the FDA to address issues the agency raised during inspections of the company’s quality system. Under the terms of this agreement, Physio-Control was permitted to ship a limited number of products to emergency care providers to meet public health and safety needs until quality system improvements were completed.
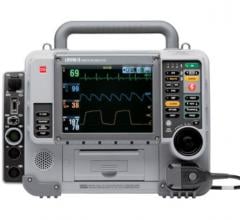
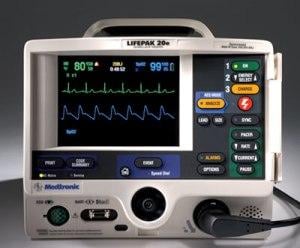
During the past two years, Physio-Control received FDA 510(k) clearance of the LIFEPAK 15 monitor/defibrillator, the LIFEPAK 20e defibrillator/monitor and the LIFENET System Version 4.1, the latest update of its Web?based platform for capturing and sharing emergent STEMI patient data. The company also introduced a new battery operated version of the LUCAS Chest Compression System, a portable, easy-to-use device that delivers automated chest compressions to improve blood flow in victims of cardiac arrest.
For more information: www.physio-control.com


 January 13, 2026
January 13, 2026