October 12, 2016 — VIVA (Vascular Interventional Advances) Physicians announced a number of highly anticipated late-breaking clinical trial results at VIVA 16, hosted at the Wynn Las Vegas Sept. 18-22.
Below are highlights of the presentations:
MOBILE Clinical Trial: Marrowstim PAD Kit for the Treatment of Critical Limb Ischemia in Subjects with Severe Peripheral Arterial Disease
Presenter: Michael P. Murphy, M.D.
The phase 3 MOBILE clinical trial is a prospective, double-blind, placebo-controlled, randomized, multicenter study that sought to determine if autologous bone marrow–derived progenitor cells could decrease major amputation in patients with critical limb ischemia. It enrolled 152 patients at 24 sites. The rationale and design of this trial was based on a previous phase 1 trial and used a 3 (treatment) to 1 (placebo) randomization scheme; stratification of randomization was also based on Rutherford score and presence of diabetes. This is the first phase 3 trial in the United States in cell therapy.
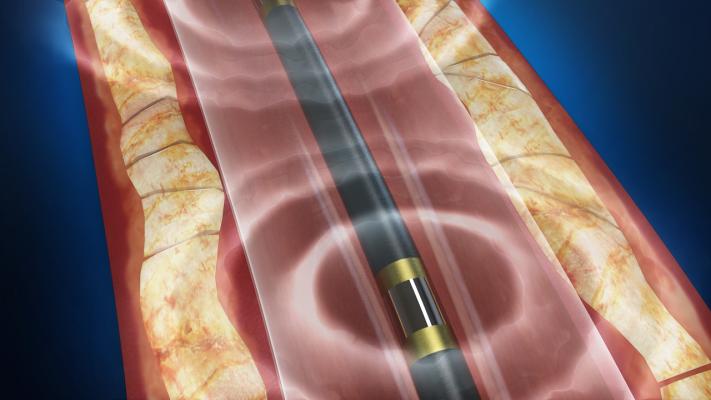
Safety and Performance of the Shockwave Lithoplasty System in Treating Calcified Peripheral Vascular Lesions: Six-Month Results from the Two-phase DISRUPT PAD Study
Presenter: Prof. Thomas Zeller, M.D.
Lithoplasty technology integrates lithotripsy with balloon-based interventional devices. Built on a traditional balloon catheter platform, lithoplasty devices use the intermittent pulsatile mechanical energy of lithotripsy to disrupt both superficial and deep calcium while minimizing soft tissue injury and an integrated balloon to dilate lesions at low pressures, restoring blood flow.
The DISRUPT PAD study is a single arm, two-phase multicenter study that enrolled 95 patients with symptomatic calcified femoropopliteal lesions ≤ 15 cm in length. Clinical data from the study demonstrate compelling safety, consistent procedural success across all patient subgroups, high acute gain and minimal acute vessel injury. Midterm results show sustained patency, target lesion revascularization and functional improvement through six months. There were no major amputations, perforations, thrombus or distal embolization events.
IN.PACT SFA Randomized Trial: Drug-coated Balloons Show Superior Three-Year Outcomes Versus Standard Angioplasty
Presenter: Prakash Krishnan, M.D.
IN.PACT SFA is a prospective, multicenter, randomized, single-blinded trial that enrolled 331 patients with symptomatic femoropopliteal lesions. Patients were randomly assigned in a 2:1 ratio to treatment with the In.Pact Admiral DCB (Medtronic) or percutaneous transluminal angioplasty (PTA).
At three years, patients treated with the In.Pact Admiral DCB demonstrated significantly superior primary patency when compared to PTA. In this multicenter randomized trial, the In.Pact Admiral DCB was shown to have superior long-term patency and low reintervention rates when compared to angioplasty. To date, this is the only DCB to show durable treatment effect through three years, supporting its continued use as a first-line treatment for symptomatic femoropopliteal disease.
VIRTUS Iliofemoral Stenting U.S. IDE Study
Presenter: Stephen Black, M.D.
VIRTUS is a prospective, multicenter, single-arm, nonrandomized global study that assesses the safety and efficacy of the Vici venous stent system (Veniti Inc.) in achieving patency of the target venous lesion in patients with clinically significant, chronic, nonmalignant occlusion of the iliofemoral venous tract. A total of 200 patients are being enrolled in clinical centers worldwide: 30 feasibility subjects and 170 pivotal.
Data suggest intravascular ultrasound (IVUS) may be more effective than venography in defining lesion severity. In the VIRTUS feasibility cohort analyzed, preprocedure venogram and IVUS measurements of minimum luminal diameter (MLD) were highly correlated. Post-stent percentage stenosis was not highly correlated between venography and IVUS. The degree of stent oversizing was greater by venogram than IVUS. Venography is important for guiding appropriate stent deployment, although IVUS may be more accurate for assessing the severity of a lesion and for proper venous stent sizing.
Drug-coated Balloon Treatment for Patients with Intermittent Claudication: Insights from the IN.PACT Global Full Clinical Cohort
Presenter: Michael Jaff, DO
IN.PACT Global is a multicenter, international, prospective, single-arm study designed to expand the clinical evidence of the In.Pact Admiral DCB (Medtronic) in the treatment of patients with symptomatic femoropopliteal peripheral peripheral artery diesease. A total of 1,406 patients were treated with the In.Pact Admiral DCB and analyzed as part of the consecutively enrolled clinical cohort. A subset of the clinical cohort subjects were required to undergo duplex ultrasound imaging at 12 months and at the time of any reintervention within 12 months to assess target lesion patency. The primary effectiveness endpoint, freedom from clinically driven target lesion revascularization within 12 months was 92.6 percent. These results confirm safety and effectiveness of the In.Pact Admiral DCB in femoropopliteal lesions. To VIVA Physicians’ knowledge, this is the largest clinical evaluation of real-world patients treated with DCBs to date.
For more information: www.vivaphysicians.org


 November 14, 2025
November 14, 2025 









