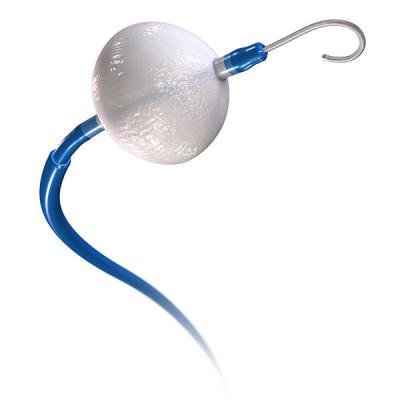
April 10, 2017 — Medtronic recently announced the first enrollments in the STOP Persistent AF clinical trial. The study will evaluate the safety and effectiveness of a pulmonary vein isolation-only (PVI) strategy for treating patients with persistent atrial fibrillation (AF), using the Arctic Front Advance Cardiac CryoAblation Catheter. John Harding, M.D., Doylestown Hospital in Doylestown, Penn., treated the first patient enrolled in the trial.
STOP Persistent AF is a prospective, multicenter, single-arm clinical trial that will enroll up to 225 patients at 25 centers in the United States, Canada, Europe and Japan. Patients will be followed for 12 months after the initial cryoballoon ablation procedure.
"Gaining meaningful data from this trial will help further clinicians' understanding of possible treatment options for patients with persistent AF," said co-principal investigator Hugh Calkins, M.D., director of the electrophysiology laboratory and arrhythmia service at the Johns Hopkins Hospital in Baltimore. "As AF progresses and episodes become more constant, patients' quality of life diminishes while their risk of AF-related health effects, such as heart failure and stroke, increases. This trial could help us advance care for this hard-to-treat population."
AF is one of the most common heart rhythm disorders, affecting more than 33 million people worldwide.[1] In the United States, AF affects nearly 6.1 million adults, and patients with persistent AF represent approximately a quarter of all AF cases.[2,3] Persistent AF occurs when the upper chambers of a patient's heart beat erratically for more than seven days and medical intervention and drug therapy is required to stop the episode. The risk of stroke and heart failure increases in patients with AF.[4,5]
Recently updated guidelines published by the European Society of Cardiology (ESC) acknowledge cryoablation therapy for AF, and recognize PVI as an effective and preferred treatment option for select patients with AF. The Medtronic cryoballoon has been used in more than 250,000 cases worldwide. Currently, no ablation catheters are approved for treating persistent AF in the United States. The Medtronic Arctic Front Advance Cryoablation System is approved in the U.S. for the treatment of drug-refractory, recurrent, symptomatic paroxysmal atrial fibrillation and in Europe for the treatment of atrial fibrillation.
Watch a VIDEO interview with HRS President Michael Gold, M.D., from ACC.17 on recent advances in EP.
For more information: www.medtronic.com
References:
1. Chugh S, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014; 129:837-847.
2. January C, Wann L, Alpert J, Calkins H, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014.
3. Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clinical Epidemiology. 2014;6:213-220. doi:10.2147/CLEP.S47385.
4. Fuster et al. Journal of the American College of Cardiology. 2006; 48:854-906.
5. ACC/AHA/ESC Guidelines for the Management of Patients with Atrial Fibrillation


 February 06, 2026
February 06, 2026 









