Image courtesy of Medtronic
February 10, 2015 — Medtronic announced the initiation of the Cryo4 Persistent AF (Cryoballoon Ablation for Early Persistent Atrial Fibrillation) clinical trial. The trial will evaluate the 12-month clinical outcomes of cryoballoon ablation for isolating the pulmonary veins, without additional ablation strategies, using the Medtronic Arctic Front Advance Cryoballoon system to treat patients with early persistent atrial fibrillation (AF). Tillman Dahme, M.D. at the Universitäsklinikum of Ulm in Ulm, Germany enrolled the first patient in the trial.
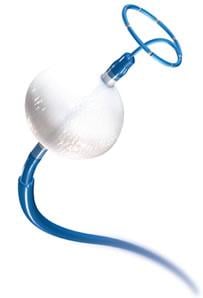
Cryoablation is currently available to European patients with AF; in the United States, cryoablation is U.S. Food and Drug Administration (FDA) approved for patients with drug refractory, recurrent, symptomatic, paroxysmal AF. In a minimally invasive procedure, the cryoballoon inflates and fills with coolant to ablate the tissue where the pulmonary veins enter the left atrium (upper chamber of the heart), blocking the signals that initiate AF.
"Cryoballoon ablation is known to provide a safe and effective treatment approach for indicated patients. Through this study, we hope to show the significant benefits of cryoballoon ablation in primary intention, as an efficient, single-shot approach to isolate the pulmonary veins in persistent AF patients, without the need for further cardiac ablations," said Serge Boveda, M.D., manager of the Cardiac Arrhythmias Department in Clinique Pasteur, Toulouse, France, and co-principal investigator in the trial.
Persistent AF occurs when the heart's upper chambers beat erratically for more than seven days and medical intervention or drug therapy is needed to stop the episode. Early persistent AF patients are those who have been diagnosed with the condition for less than a year. If left untreated, patients with AF have up to a five times higher risk of stroke and an increased chance of developing heart failure.
"We work to provide the most innovative therapies available to physicians worldwide for the treatment of atrial fibrillation, which is expected to affect even more patients over the next few decades," said Reggie Groves, vice president and general manager of the AF Solutions division at Medtronic. "An advanced therapy that is consistent and reproducible, cryoablation has the potential to effectively treat even more AF patients, providing them with improved quality of life."
The Cryo4 Persistent AF trial is a multicenter, prospective, non-randomized, post-market study that will enroll up to 130 patients at 10 sites in France and Germany. Trial participants will be followed for 12 months after treatment for signs of adjudicated AF, atrial flutter or atrial tachyarrhythmias greater than 30 seconds.
Treatment with the Medtronic Arctic Front Advance Cryoballoon System involves a minimally invasive procedure that freezes the tissue where the pulmonary veins enter the left atrium, to block the electrical signals that trigger erratic heart rhythms. The system has been used to treat more than 100,000 patients in approximately 900 centers across 45 countries.
For more information: www.medtronic.com


 January 05, 2026
January 05, 2026 









