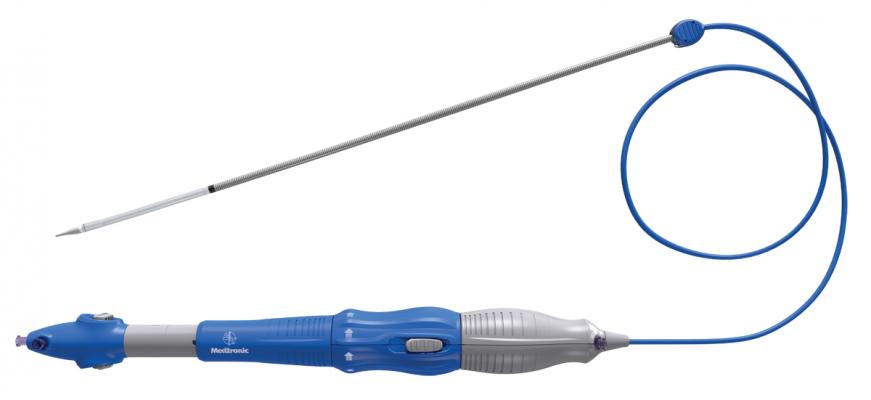
February 9, 2015 — Medtronic announced CE (Conformité Européene) mark for the 26 and 29 mm sizes of the CoreValve Evolut R System, a next-generation self-expanding valve for transcatheter aortic valve implantation (TAVI) providing the option to recapture and reposition the valve during procedures. The new larger valve sizes are delivered through a 14 French equivalent delivery system allowing small vessels to be accessed, and offer an extended sealing skirt intended to further promote valve sealing at the annulus.
The 23, 26 and 29 mm sizes of the CoreValve Evolut R and the CoreValve EnVeo R Delivery Catheter System are available in Europe and other countries that recognize the CE mark. It is not approved for commercial use in the United States, where it is currently undergoing clinical trials.
“The clinical experience with the Evolut R system has been exceptional. The system’s optimal positioning and delivery of the valve are essential to achieve the very best clinical results in TAVI. Evolut R’s low profile, positioning accuracy and enhanced sealing, all supported with the option to recapture and reposition during implantation, are a significant advance for transcatheter therapy,” said Ganesh Manoharan, M.D., consultant cardiologist, Royal Victoria Hospital, co-lead for the CoreValve Evolut R CE mark study slated for presentation at the 2015 American College of Cardiology (ACC) annual meeting. “Expanding the Evolut R valve size range enables physicians to bring these important advances to a much broader range of patients.”
The system is designed for first-time positioning accuracy and also offers an InLine Sheath that significantly reduces the profile to a 14 French equivalent, less than 1/5 inch; a smaller profile size provides a greater opportunity to treat patients with smaller vessels (down to 5 mm), which may minimize the risk of major vascular complications in some patients. The Evolut R valve is also anatomically designed for optimal fit and sealing, while maintaining strong hemodynamic performance.
For more information: www.medtronic.com


 December 24, 2025
December 24, 2025 









