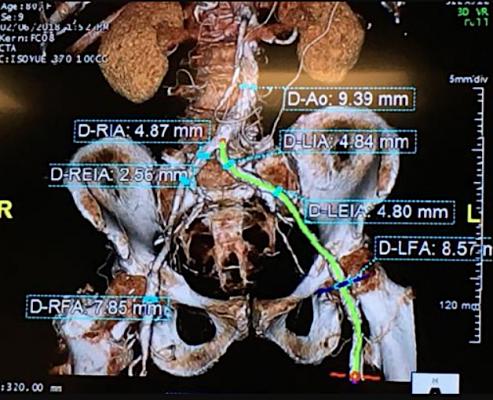
An example of a 80-year-old TAVR patient planning CT scan from Northwestern Medicine's Central DuPage Hospital. This was used in a heart team meeting with the TAVR staff, including a cardiac surgeon and interventional cardiologist, to determine if this patient was eligible for TAVR, the best access routes to deliver the valve, device valve size needed, and to look for any potential anatomical issues so they can be planned for.
September 2, 2020 — Northwestern Medicine Bluhm Cardiovascular Institute physicians recently completed their 2,000th transcatheter heart valve procedure, a milestone innovation that has shifted valve repair and replacement from operating room to catherization lab, dramatically reducing procedural time, recovery time and mortality risk for thousands of patients with heart valve disease.
Northwestern Memorial Hospital was one of a select group of American hospitals utilizing this technology in the earliest stages of trial and discovery and continues to be active in clinical trials for minimally invasive aortic, mitral and tricuspid valve interventions today. Physicians at Northwestern Medicine Central DuPage Hospital, who recently completed their 200th transcatheter valve replacement, also contributed to the 2,000th milestone.
“I’m feeling great,” said Edith Burns, who on August 24 underwent placement of a MitraClip, a device directed to the heart on a catheter where it is then positioned to “clip,” or join two parts of the mitral valve together, eliminating backward flow of blood through the valve that can cause severe fatigue, shortness of breath and chest pain. “In fact I can’t believe how good I feel right now less than a day after the procedure. I already went for a walk.”
Northwestern Memorial Hospital performs the highest volume of transcatheter and surgical valve procedures in Illinois, having completed the most aortic, mitral and tricuspid valve procedures in the state. Northwestern’s research activities in this area have grown tremendously since the first procedure in 2008. Currently, there are 18 transcatheter valve trials, including five early feasibility studies, the critical first clinical step of research and development, and the first-in-human transcatheter trials for the tricuspid valve.
“This milestone could not have been reached without our patients, who put their trust in us as we worked together to pioneer a better way to treat heart valve disease,” said Patrick McCarthy, M.D., executive director of the Bluhm Cardiovascular Institute and chief of cardiac surgery at Northwestern Memorial Hospital. “This is what Bluhm Cardiovascular Institute is all about — combining research and exceptional outcomes for our patients, while offering them the most advanced options for treating their cardiovascular disease.”
In transcatheter aortic valve replacement (TAVR), the new valve is placed on a catheter, or tube, which is guided up to the patient’s heart via an artery as the doctor directs the position with X-ray guidance. The new valve is placed into the diseased valve and opened like an umbrella, pushing aside the old valve and providing a new, clear pathway for blood flow through the valve. In open-heart surgery, the traditional method for valve repair and replacement, the chest is opened to provide access to the unhealthy valve.
After transcatheter valve replacement, patients are typically recovering in the hospital for two days, versus five days after open-heart surgery. Early complications are generally low, and the valves have proven durable.
Annually, cardiovascular disease kills more Americans than any other disease, including all cancers combined, making this effective, lower risk option highly desirable.
“While open-heart surgery remains the gold standard procedure for many patients, especially young patients and those with a leaky mitral valve, minimally invasive transcatheter heart valve repair or replacement is considered a sea change in the field of cardiology” said McCarthy, the Heller-Sacks Professor of Cardiothoracic Surgery at Northwestern University Feinberg School of Medicine.
Charles Davidson, M.D., clinical chief of cardiology at Northwestern Memorial Hospital and the vice chair for clinical affairs in the Department of Medicine at Feinberg, performed the 2,000th procedure, the MitraClip on Burns. He was joined by James Flaherty, M.D., medical director of the coronary care unit at Northwestern Memorial Hospital, and Andrei Churyla, M.D., cardiac surgeon at Northwestern Medicine.
Davidson also performed the first transcatheter aortic valve replacement with McCarthy and S. Chris Malaisrie, M.D., cardiac surgeons at Northwestern Memorial, and the 500th transcatheter aortic valve replacement on July 23, 2016.
Since the first transcatheter aortic valve replacement at Northwestern, the device is smaller, making it easier for physicians to deliver and implant the valve. This expanded the technology to smaller patients and those with vascular disease of the legs. The time for the average procedure has shifted from several hours in 2008 to usually around one hour in 2020. Also, at Northwestern Memorial, the procedure is mostly performed without general anesthesia allowing for a faster recovery. As the technology and experience has grown, recent studies have demonstrated superiority to open heart surgery for aortic stenosis.
“At Northwestern, we have been pioneering transcatheter valve therapy since its inception and we are still innovating the application of this technology,” Davidson said. “Transcatheter trials are also transforming the treatment of mitral and tricuspid valve disease. Aortic valve replacement has added years and quality to countless patients’ lives and offers hope for others as we continue to work on effective nonsurgical therapies.”
Other notable transcatheter valve moments at Northwestern Memorial Hospital include:
• TAVR performed on a 101-year-old Peoria woman, whose family credited the procedure with extending her length and quality of life when she passed away at 108 years old.
• A New England Journal of Medicine study published in 2017 and co-authored by Dr. Malaisrie showed patients who were at low risk for surgical complications benefited significantly from TAVR. The findings were the results of the PARTNER 3 trial.
First in Illinois and one of first in the nation to use new mitral and tricuspid replacement valves
• Currently enrolling in multiple clinical trials for transcatheter tricuspid valve replacement or repair to treat tricuspid regurgitation, the next wave of transcatheter valve innovation
• Leaders in the COAPT clinical trial of the MitraClip device for heart failure patients
• Leaders in the recently approved REPAIR MR clinical trial of the efficacy of MitraClip compared to surgery for moderate risk and elderly patients with mitral valve prolapse
“Through our continued work in the Clinical Trials Unit, we are advancing the field of cardiovascular medicine,” Malaisrie said. “This fast-changing field of medicine is only getting better.”
Northwestern Expands TAVR to Suburban Hospitals
Shortly after the merger of Northwestern Memorial and Central DuPage hospitals in 2015, Bluhm Cardiovascular Institute physicians from both sites combined to start a transcatheter aortic valve replacement program at Central DuPage to provide closer-to-home access to this technology to patients in the western suburbs. Central DuPage physicians have completed more than 200 TAVRs with zero mortality. Next, the hospital will begin transcatheter mitral valve repair with the MitraClip device.
TAVR is also available at Northwestern Medicine McHenry Hospital, where a new surgical team recently formed to provide increased access to the procedure for patients in Chicago’s far northwest suburbs.
Northwestern Medicine’s Bluhm Cardiovascular Institute is one of the top 10 national programs for cardiology and heart surgery, according to U.S. News and World Report, and ranked the top cardiovascular program in Illinois and the surrounding states for more than 10 consecutive years.
Related Northwestern Medicine TAVR Content:
Hospital Consolidation May Increase Access to TAVR, New Cardiac Technologies
VIDEO: How Consolidation Into Larger Health Systems Can Improve Access to TAVR
VIDEO: Cath Lab Tour at Northwestern Medicine's Central DuPage Hospital
Northwestern Medicine Mobile Stroke Unit Delivers Life-Saving Care 30 Minutes Sooner
360 View of a Cath Lab at Northwestern Medicine Central DuPage Hospital


 December 24, 2025
December 24, 2025 









