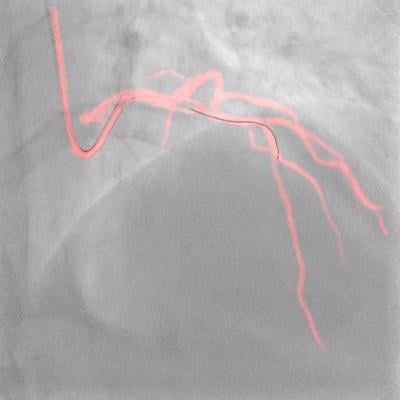
Philips Dynamic Coronary Roadmap
May 16, 2023 — Royal Philips, a global leader in health technology, announced late-breaking data from the DCR4Contrast (Dynamic Coronary Roadmap for Contrast Reduction) trial, the largest ever randomized controlled multicenter clinical trial to investigate the ability of Philips Dynamic Coronary Roadmap (DCR) to reduce the total iodinated contrast media volumes administered during percutaneous coronary intervention (PCI) procedures, compared to PCI performed without DCR guidance. The results were presented today at the European Association of Percutaneous Cardiovascular Interventions (EuroPCR) 2023 course and will also be presented later this week at the Society for Cardiovascular Angiography & Interventions (SCAI) 2023 scientific sessions.
The number of high-risk patients undergoing PCI procedures worldwide has increased significantly [2]. Iodine contrast media is used to visualize the coronary arteries during PCI procedures, but it can potentially harm the kidneys, risking the development of contrast-induced acute kidney injury (CI-AKI) after the procedure. The study found that Dynamic Coronary Roadmap reduced the total iodine contrast volume per procedure on average by 28.8% (95% Confidence Interval: 18.9%, 38.2%), and reduced the number of angiograms per procedure on average by three runs based on a procedure with an average of 11 runs or 26.3% reduction (95% Confidence Interval: 16.8%, 35.1%) [1].
“Enabling physicians to decrease contrast administration during procedures with tools like DCR could make a significant contribution to both the safety and quality of PCI. This is important at a time when PCI is increasingly used in patients with a complex clinical or anatomical profile,” said Prof. Javier Escaned, MD, Head of the Interventional Cardiology Section at Hospital Clinico San Carlos, Madrid Spain, who presented the results today during a late-breaking session at the annual meeting for the EuroPCR 2023 (May 16-19, Paris, France).
“At Philips, we are committed to championing technologies that improve the patient’s experience, improve their safety and expand access to procedures to new patient groups. Dynamic Coronary Roadmap helps achieve these goals by structurally reducing the amount of contrast agent required for PCI procedures,” said Dr. Atul Gupta, Chief Medical Officer for Image Guided Therapy at Philips.
Multi-center, prospective, unblinded, stratified, 1:1 randomized controlled trial
The DCR4Contrast trial ran from November 2019 to February 2023 across hospitals in the U.S., Europe and Israel. In total, 371 patients were randomized and stratified within both ad hoc and planned PCI, according to the number of vessels to be treated. Patients in the DCR group underwent PCI procedures where DCR was used to guide coronary wires, balloons, stents and other PCI diagnostic or therapeutic devices. Patients assigned to the control group underwent PCI without DCR support, following the current standard of care [1].
On Thursday, May 18 Professor John C. Messenger, MD, University of Colorado School of Medicine and Director of the Cardiac Catheterization Laboratories and Cardiovascular ICU at the University of Colorado Hospital will present the data at the Society for Cardiovascular Angiography & Interventions (SCAI): “These results are very exciting,” Professor Messenger commented. “They confirm that Philips Dynamic Coronary Roadmap can be applied to all PCIs and has the potential to increase overall PCI safety by mitigating one of the few preventable contributors (iodinated contrast media use) to contrast-induced acute kidney injury following PCI.” More information on Professor Messenger’s presentation at SCAI can be found here.
Philips’ Dynamic Coronary Roadmap software is a real-time visualization innovation that removes the need for additional contrast media injections by overlaying the angiogram onto real-time motion-compensated 2D fluoroscopic imaging to provide interventionalists with continuous visual feedback on the positioning of guide wires and catheters. Complementing this technology, the Philips IntraSight precision guidance system streamlines lesion assessment, simplifies vessel sizing, enables precise therapy delivery and also supports the operator in their goal to reduce contrast volumes.
For more information: www.philips.com
Find more SCAI23 conference coverage here
Find more EuroPRC23 conference coverage here
References:
[1] Study reference number DHF287327
[2] Waldo SW, Gokhale M, O’Donnell CI, Plomondon ME, Valle JA, Armstrong EJ, Schofield R, Fihn SD, Maddox TM. Temporal Trends in Coronary Angiography and Percutaneous Coronary Intervention: Insights From the VA Clinical Assessment, Reporting, and Tracking Program. JACC Cardiovasc Interv 2018;11:879–888.


 February 19, 2026
February 19, 2026 









