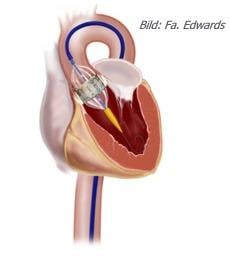
May 9, 2011 — John Webb, M.D., FSCAI, has traveled the globe teaching fellow physicians how to repair or replace faulty heart valves using minimally invasive techniques. In a keynote Founders’ Lecture delivered at the Society for Cardiovascular Angiography and Interventions (SCAI) 2011 Scientific Sessions last week, he recount the challenges that arose in developing valve therapies that avoid open-chest surgery. Instead, interventional cardiologists use slender tubes, or catheters, that are threaded into the heart through blood vessels.
“When we started, we thought it was very possible that a transcatheter valve procedure could be developed and could be reproducible, but with no experience, nobody really knew for sure,” said Webb, the McCleod Professor of Heart Valve Intervention at the University of British Columbia in Vancouver.
In his Founders’ Lecture, Webb focused primarily on catheter procedures used to implant new aortic valves, the valve that controls the flow of blood from the heart to the rest of the body. He also addressed the development of transcatheter implantation of pulmonary valves (between the heart and lungs) and mitral valves (between the upper and lower chambers on the left side of the heart). In addition, Webb presented advances in catheter techniques for implanting replacement valves inside artificial valves that have failed after open-chest surgery.
The first catheter procedures to treat constricted aortic valves were being performed just as Webb was beginning his training in interventional cardiology. He became interested in how to improve the technique by doing more than just stretching the calcified aortic valve with a balloon. But the idea of an implantable valve was dismissed by skeptics as too far-fetched.
Refining the technique took many years, said Webb, who has since performed or mentored more than a thousand catheter-based aortic valve replacements in 20 countries.
“With time, as each new problem was identified, we were able to take a careful look at it and analyze it and come up with a way of managing these patients so that these complications would be less likely,” he said. “It was really a story of incremental improvements in the technique and patient selection and the device.”
It was not until about 2006 that Webb became convinced that implantation of an aortic valve with a catheter would become a common procedure. Though practical obstacles still exist, he is confident they can be overcome. He continues to welcome cardiovascular teams to the University of British Columbia for intensive training in the procedure.
For more information: www.scai.org


 December 24, 2025
December 24, 2025 









