August 4, 2021 — Temporary Spinal cord stimulation (SCS) was effective in suppressing post-operative atrial fibrillation (AF) after coronary artery bypass (CAGB) surgery CABG without any adverse events in a randomized pilot study. The TEmporary Spinal CoRd StiMulatIon to PrevenNt Atrial FibrillaTION AFter Cardiac Surgery (TerminationAF) study was presented as a late-breaking study presented at the 2021 Heart Rhythm meeting, the annual conference of the Heart Rhythm Society (HRS).
Spinal cord stimulation has been shown to be effective in the treatment of chronic pain and intractable angina pectoris. Recently, animal studies have demonstrated that SCS can also suppress AF, explained presenter Alexander B. Romanov, M.D., Ph.D., FHRS, E. Meshalkin National Medical Research Center of the Ministry of Health, Novosibirsk, Russia. He said this study looked at its application in human patients who underwent CABG to see if it could suppress AF in this patient population.
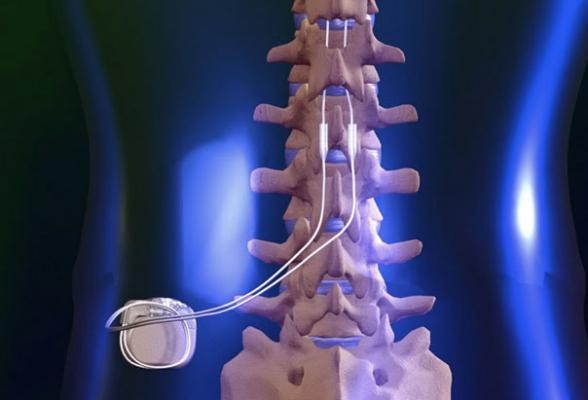
The study included 52 patients with indications for coronary artery bypass grafting (CABG) and history of paroxysmal AF were randomized to 2 groups: CABG plus standard medical therapy (MED) with beta-blockers (n=26, Control group) and CABG plus MED plus percutaneous lead placement for temporary SCS (n=26, SCS group). In the SCS group under local anesthesia and with fluoroscopic guidance, temporary leads were placed at C7-T4 level according to patient’s sense of paresthesia and connected to a SC stimulator externally fixed on patient’s chest. Temporary SCS was begun 3 days before elective CABG, deactivated during surgery, reactivated in the intensive care unit after CABG, and continued for 7 days at which time the leads were removed Continuous external ECG monitoring was performed for 30 days after CABG in all patients. These primary objectives were tested over the 30-day post-operative period: 1) occurrence of adverse events, including death, stroke or TIA, myocardial infarction and kidney injury; and 2) occurrence of AF or any atrial tachyarrhythmia lasting ≥ 30 seconds.
Percutaneous lead placement for temporary SCS was successfully performed in all 26 patients before CABG without any complications. There were no any adverse events related to temporary SCS in any patient throughout follow-up. There were no significant differences in CK-MB and creatinine levels between groups (p=0.1 and 0.2, respectively) as well as other typical CABG-related complications (p>0.05). Post-operative AF occurred in 8 (30.7%) of
26 patients in the Control group versus only 1 (3.8%) of 26 patients in the SCS group (p=0.012, log-rank test, Figure).
Researchers said further studies of SCS with larger samples should be conducted to test its clinical value as a peri-operative intervention.
Find additional HRS 2021 late breaking trials
Find more HRS 2021 conference news


 December 19, 2025
December 19, 2025 









