April 6, 2018 — University Hospitals Harrington Heart & Vascular Institute has officially opened the APOLLO trial, implanting the first Intrepid transcatheter mitral valve replacement system in the study. Alan Markowitz, M.D., and Guilherme F. Attizzani, M.D., performed the procedure, the first in the state of Ohio.
As part of the APOLLO trial, UH patients with severe symptomatic mitral regurgitation will have the opportunity to be randomized to receive either conventional open-heart mitral valve replacement surgery or an investigational transcatheter mitral valve replacement (TMVR). In addition, patients who are not good surgical candidates could have the opportunity of being treated with TMVR.
According to Attizzani, UH interventional cardiologist and co-director, Valve and Structural Heart Disease Center, UH Harrington Heart & Vascular Institute, the APOLLO trial is a welcome development for patients with mitral valve disease.
“There is currently no TMVR technology approved by the FDA [U.S. Food and Drug Administration] and this trial may open a new, very important avenue on the field,” said Attizzani, who is also assistant professor of medicine at Case Western Reserve University School of Medicine. “The only percutaneous technologies we currently have to treat mitral insufficiency are MitraClip, which enables percutaneous native mitral valve repair, and transcatheter valves used for valve-in-valve procedures. This study is, therefore, extremely important as it may lead to a paradigm shift in terms of the treatment of native mitral valve insufficiency.”
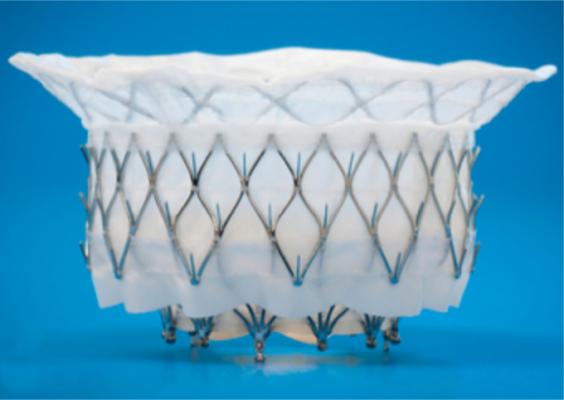
The APOLLO trial is testing the Intrepid TMVR system, which is compressed inside a hollow delivery catheter and is inserted between the ribs to enter the heart. The new replacement valve is expanded directly into the malfunctioning mitral valve. The outer stent frame is designed to attach and conform to the native valve without the need for additional sutures, tethers or anchors to secure the prosthesis. The inner stent houses the valve, which is made from bovine tissue and is intended to maintain blood flow.
The Intrepid valve is implanted using a transapical approach through the apex. However, the technology is expected to evolve and become even less invasive.
“Today, surgeons need to open a small hole in the apex of the heart, then the valve is advanced through it and implanted,” said Markowitz, chief surgical officer, and director, Valve and Structural Heart Disease Center, UH Harrington Heart & Vascular Institute, and clinical assistant professor of surgery, CWRU School of Medicine. “It’s a beating-heart procedure without the heart-lung machine. Industry is, however, already working on a technology that is going to be transseptal (i.e., can be done via puncture of the femoral vein). This will further reduce the invasion to the patient by not having to go through the apex.”
UH is the only healthcare system in Northeast Ohio to give its patients access to the APOLLO trial.
“In addition to the APOLLO trial, we offer MitraClip therapy, an FDA-approved therapy for mitral valve insufficiency,” said Markowitz. “We also perform valve-in-valve and other complex procedures. It’s the full spectrum of mitral valve interventions, a combined effort of interventional cardiology and cardiac surgery.”
Once the APOLLO trial is completed, Attizzani expects patients with mitral valve disease to have more options to treat mitral valve disorders.
“Although it is impossible to predict the trial results, my expectation is that the TMVR system will be shown to be safe and effective,” said Attizzani. “If it is demonstrated to be non-inferior to surgical mitral valve replacement, it will be a key step in terms of the potential future approval of this novel technology by the FDA, ultimately enabling more patients to have the option of an effective and less invasive procedure.”
For more information: www.medtronic.com


 December 24, 2025
December 24, 2025 









