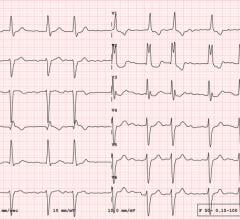
The first of Vektor Medical's new studies, being presented at HRS 2023, showcases vMap’s utility in unstable ventricular tachycardia (VT) ablation. (Graphic: Business Wire)
May 18, 2023 — Vektor Medical, pioneers of technology to accurately map arrhythmias using only 12-lead ECG data, unveiled two studies further demonstrating clinical utility. The first study showcases vMap’s utility in unstable ventricular tachycardia (VT) ablation, sometimes called ‘unmappable VT’. The second study, a proof-of-concept effort, demonstrated a brand-new opportunity to map Atrial Tachycardia using 12-lead ECG data obtained in the outpatient setting. Results will be presented at the Heart Rhythm Society’s Annual Conference 2023 in New Orleans.
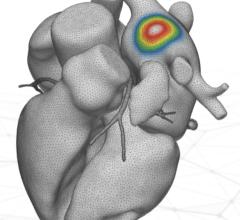
vMap, an FDA-cleared electrophysiology device, utilizes advanced machine learning techniques and proprietary computational models to extract functional insights for accurate and efficient arrhythmia source location from a standard 12-lead ECG, without requiring invasive mapping techniques.
The first study, ‘Prospective Real-Time Use of Forward-Solution ECG Mapping to Facilitate Focused Activation Mapping of Unstable Ventricular Tachycardia (VT): Accuracy and Outcomes,’ demonstrated the accuracy and utility of vMap for mapping hemodynamically unstable VT during catheter ablation procedures. Out of 16 consecutive patients who underwent VT ablation, seven of the eight patients undergoing prospective ECG mapping had hemodynamically unstable VT. All seven VT patients had successful activation mapping guided by vMap. The results demonstrated a path forward for mapping these ‘unmappable’ VT while indicating an improvement in patient outcomes and further validating vMap’s accuracy for arrhythmia source identification, including:
- 98% reduction in total VT episodes during mean 4.2 month follow-up
- Mean accuracy of ECG mapping was 1.2±0.8cm when compared to invasive activation for all 16 patients
The second proof-of-concept study demonstrated vMap’s utility with outpatient 12-lead ECGs to accurately identify an Atrial Tachycardia (AT) arrhythmia source location. In the study, “Proof-of-Concept Forward-Solution Mapping of a Focal Atrial Tachycardia Origin Using the Outpatient 12-Lead Electrocardiogram,” the outpatient ECG was loaded into vMap and target AT locations were identified with the computational modeling software. The arrhythmia source location was then confirmed using invasive mapping techniques. Additional validation is ongoing in larger populations, but early results demonstrated:
- Agreement between vMap’s non-invasively identified location at the anterior base of the left atrial appendage as the highest probability focal AT source, with the location identified by invasive electron anatomical mapping techniques.
- The arrhythmia source accuracy of the vMap as compared to invasive mapping was 8.8mm as defined by center-to-center spatial distance between the sites of early activation.
“Arrhythmia source mapping is a crucial component of targeted catheter ablation procedures for complex procedures and helps us to ensure effective and durable therapy,” commented Dr. Gordon Ho, Electrophysiologist at UC San Diego Health and lead author of the VT study. “vMap provides actionable information about where the sources most likely are to help drive procedural efficiency in challenging cases such as unstable VT and complex AF. I’ve been impressed with how easy the system is to incorporate into our workflow and the value it adds to more quickly identify target lesion sites.”
The results add to the growing body of evidence that demonstrates vMap’s utility for the successful treatment of a wide range of arrhythmias.
For more information: www.vektormedical.com
Find more HRS conference coverage here


 July 30, 2024
July 30, 2024