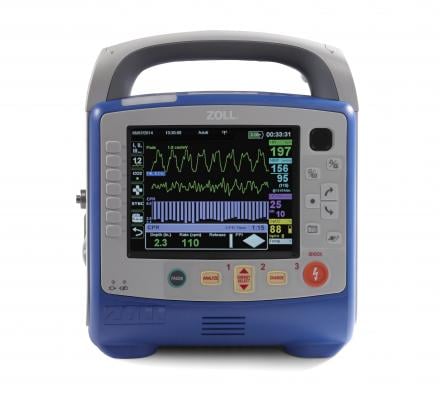
October 16, 2017 — Zoll Canada, a subsidiary of Zoll Medical Corp., announced it has won the tender to equip all ambulances in the Province of Quebec with the Zoll X Series Monitor/Defibrillator. The purchase of 866 X Series was negotiated by GACEQ (Groupe d’approvisionement en commun de l’Est Qué).
The X Series weighs just 5.3 kilograms (less than 12 pounds), and is about twice as light as what most paramedics carry today, according to the company. It is built on an expandable platform that delivers the capabilities one would expect from a full-featured monitor and is designed for use with all patients, ranging from neonates through adults. This state-of-the–art monitor is equipped with WiFi, faster at measuring vital signs and better at measuring carbon dioxide levels.
CPR support technology on the X Series includes Real CPR Help, See-Thru CPR and the CPR Dashboard. These technologies include the capacity to capture and analyze CPR quality, an important and integral component to improving outcomes from sudden cardiac arrest. High-quality CPR is crucial to patient survival from sudden cardiac arrest.
These technologies will enable Province of Québec paramedics to know in real-time if they are pushing hard enough and fast enough to meet American Heart Association (AHA) CPR guidelines, and also know how long they are off the chest between pauses in chest compressions.
For more information: www.zoll.com


 January 13, 2026
January 13, 2026 









