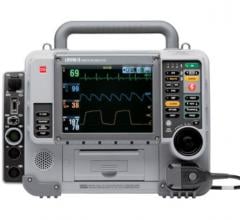
March 9, 2009 - The Ministry of Health for the Province of Quebec has awarded ZOLL Medical Corp. a contract valued in excess of $12 million to equip all ambulances in the Canadian province with the ZOLL E Series defibrillator, representing the first time the Province of Quebec will standardize its entire system to one model of defibrillator.
Under the terms of the contract, approximately 700 E Series defibrillators, along with CPR stat-padz will be purchased. The transition to ZOLL will be made region by region, 16 regions in total, with the first order already having been placed by Urgences-Santé, the EMS provider for the city of Montreal.
According to ZOLL, The Ministry of Health and Social Services wanted to standardize its resuscitation technologies by offering advanced, high-performance equipment in order to increase the quality of services offered in pre-hospital care for victims of sudden cardiac arrest (SCA). By standardizing on the ZOLL E Series, the province’s medical directors will be able to use and compare similar patient data. The level of care that will be offered will reportedly be compatible with the American Heart Association (AHA) and the Heart and Stroke Foundation’s Guidelines that place significant emphasis on CPR.
The ZOLL E Series is designed to meet the specific demands and extreme conditions that professional rescuers face every day. The E Series offers multiple data transmission options to a variety of destinations, including transmission of 12-lead ECGs to hospitals, which international guidelines now recommend for out-of-hospital use to help reduce time to perfusion in S-T segment elevation myocardial infarction (STEMI) patients.
Real CPR Help technology in all of ZOLL’s devices instantly provides first responders and advanced paramedics with technology to see how well they are performing the rate and depth of CPR chest compressions. See-Thru CPR is designed to minimize interruptions in CPR that effect resuscitation success.
For more information: www.zoll.com.


 January 13, 2026
January 13, 2026