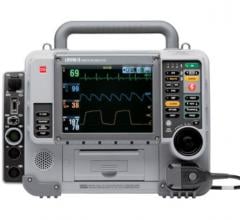
December 18, 2008 - ZOLL Medical Corp. this week received FDA 510(k) clearance to market and sell the ZOLL R Series Code-Ready clinical defibrillator with a WiFi option that allows wireless communication between the defibrillator and standard hospital networks to help ensure code-readiness and download of patient data.
Based on standard 802.11b WiFi technology, R Series defibrillators equipped with WiFi automatically send an alert when their state of readiness is compromised. Early notification maximizes patient safety by letting the clinician intervene before the defibrillator is needed for a code.
While defibrillators rarely fail, their readiness can be compromised if someone forgets to plug it in, a cable is missing, or the electrodes are old and dried out. With WiFi, when the state of readiness is compromised, a notification identifies the contributing factors and code readiness can be restored with unmatched efficiency by troubleshooting from any PC on the hospital network.
For downloading patient data, the Defibrillator Summary is an important part of the code, and the R Series WiFi capability simplifies merging the Summary Report with event documentation. At the end of the code, the summary report can be transmitted over a standard wireless network to a server hosting the CodeNet system, where it is merged and time-synchronized with the Event Report documented on CodeNet Writer.
The R Series is a Code-Ready device that is simple, smart, and ready to use. It offers a OneStep system to simplify and speed up deployment of pacing and defibrillation therapy. The R Series can also verify the condition and expiration date of the electrode set. All of this testing occurs without disconnecting electrodes or paddles, or requiring additional equipment to test shock delivery. The system provides a printed or electronic log to alert hospital personnel of any concerns in advance of a code. A simple green checkmark indicates that the R Series is fully ready for use.
CodeNet is the first software system that allows hospital teams to better document, manage, and review cardiac arrest event and resuscitation information. It brings new improvements and efficiencies in data capture, event time synchronization, and case and aggregate reporting. It is the only system that time stamps logged events and synchronizes these times with defibrillator data, providing clinicians with a complete and accurate timeline of an entire cardiac arrest event.
For more information: www.zoll.com


 January 13, 2026
January 13, 2026