March 26, 2024 — Medical imaging AI company Avicenna.AI announced that it has received 510(k) clearance from the US Food and Drug Administration for its CINA-iPE and CINA-ASPECTS products. Using a combination of deep learning and machine learning technologies, the company develops AI solutions that automatically detect and prioritize life-threatening conditions within seconds, assess them for severity, and seamlessly notify clinicians.
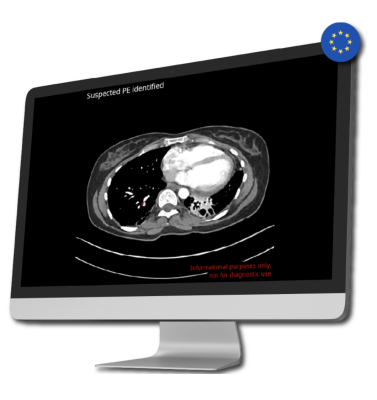
Addressing the serious issue of delayed and missed findings in diagnostic imaging, CINA-iPE is an AI-powered tool that detects incidental pulmonary embolism during routine CT scans. Unsuspected pulmonary embolism is a common finding in routine CT scans of the chest, but as little as 25% of emboli are reported during the initial interpretation. This is particularly relevant in the cancer patient population, where pulmonary embolism is a significant cause of mortality.
The CINA-iPE algorithm identifies lung blood clots detected during routine CT scans for entirely different health conditions. Scan types may include full-body scans, scans of the chest, abdomen, and pelvis, and scans of the thoracic area along with the abdomen and pelvis. The tool was validated on 381 CT scans (performed for other clinical indications than for pulmonary embolism evaluation) acquired on 39 different scanner models from five leading manufacturers, achieving excellent sensitivity and specificity.
“From day one, we have been committed to validating our AI tools on every type of CT scanner,” said Yasmina Chaibi, Clinical Affairs Manager, Avicenna.AI. “In our validation studies, CINA-iPE achieved excellent sensitivity and specificity, demonstrating its ability to provide effective prioritization and triage on routine CT scans performed for other clinical indications than pulmonary embolism suspicion.”
CINA-ASPECTS is an AI tool for stroke severity assessment, automatically processing non-contrast CT scans and calculating the “ASPECT” score, a topographic scoring system used to quantify the severity of a stroke from a CT scan of the brain. The tool computes a heat map indicating the probability of hypodensity and sulcal effacement in the brain, displays a list of infarcted regions, and provides CT images corrected from tilt to easily compare the right and left hemisphere.
In addition to assisting clinicians in the evaluation of the ASPECT score from CT scans, CINA-ASPECTS also helps improve physicians’ reproducibility in ASPECT scoring, which often varies depending on the radiologist reading the scan. The tool was rigorously validated on 200 scans acquired on 27 scanner models from four leading manufacturers.
“The validations and multi-reader-multi-case studies we conducted highlighted that CINA-ASPECTS not only obtained outstanding standalone performance, but also demonstrated that its adjunctive use significantly improved clinicians’ accuracy in the assessment of ASPECTS regions, compared to the conventional use of NCCT images alone,” added Chaibi.
CINA-ASPECTS is the first FDA-cleared tool from Avicenna.AI in the category of computer-aided diagnosis (CADx), which means it goes beyond identifying abnormalities in scans to provide an assessment of the severity of a condition.
“We are delighted to launch CINA-ASPECTS to the US market, marking a significant milestone as our first CADx product receives FDA clearance,” said Stéphane Berger, Regulatory and Quality Manager. “We take pride in being at the forefront of CADx solutions, being the first AI company supported by thorough clinical validation and a rich repository of real-world clinical data, which ensures compatibility across all manufacturers and platforms.”
CINA-iPE and CINA-ASPECTS are the latest tools from Avicenna.AI to secure FDA clearance, joining its AI solutions for medical emergencies, including automatic detection of intracranial hemorrhage (CINA-ICH), large vessel occlusion (CINA-LVO), aortic dissection (CINA-AD), pulmonary embolism (CINA-PE) from CT-scan imaging. All of Avicenna.AI’s AI tools are seamlessly integrated into radiologists’ clinical workflow, automatically triggering and reporting algorithm results through the systems already used by radiologists.
“After a long journey of dedication and perseverance, we are thrilled to announce the FDA clearance of not one, but two of our groundbreaking products,” said Cyril Di Grandi, co-founder and CEO of Avicenna.AI. “The clearance of CINA-iPE and CINA-ASPECTS marks a significant milestone in our mission to strive for excellence in advancing patient care. These achievements stand as a testament to the unwavering commitment of our team.”
For more information: www.avicenna.ai


 December 23, 2025
December 23, 2025